Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin
- PMID: 9742163
- PMCID: PMC6793026
- DOI: 10.1523/JNEUROSCI.18-19-07962.1998
Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin
Abstract
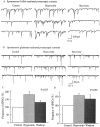
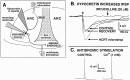
A new orexigenic peptide called hypocretin (orexin) has recently been described in neurons of the lateral hypothalamus and perifornical area. The medial and lateral hypothalamus have been loosely called satiety and feeding centers of the brain, respectively. Approximately one-third of all medial and lateral hypothalamic neurons tested, but not hippocampal neurons, show a striking nanomolar sensitivity to hypocretin. As studied with calcium digital imaging with fura-2, hypocretin raises cytoplasmic calcium via a mechanism based on G-protein enhancement of calcium influx through plasma membrane channels. The peptide has a potent effect at both presynaptic and postsynaptic receptors. Most synaptic activity in hypothalamic circuits is attributable to axonal release of GABA or glutamate. With whole-cell patch-clamp recording, we show that hypocretin, acting directly at axon terminals, can increase the release of each of these amino acid transmitters. Two hypocretin peptides, hypocretin-1 and hypocretin-2, are coded by a single gene; neurons that respond to one peptide also respond to the other. In addition to its effect on feeding, we find that this peptide also regulates the synaptic activity of physiologically identified neuroendocrine neurons studied in hypothalamic slices containing the arcuate nucleus, suggesting a second function of hypocretin in hormone regulation. The widespread distribution of hypocretin axons, coupled with the strong response to the peptide at both presynaptic and postsynaptic sites, suggests that the peptide probably modulates a variety of hypothalamic regulatory systems and could regulate the axonal input to these regions presynaptically.
Figures




References
-
- Christian EP, Dudek F. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol. 1988;59:90–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
