Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo
- PMID: 9743521
- PMCID: PMC2212528
- DOI: 10.1084/jem.188.6.1029
Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo
Abstract
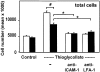
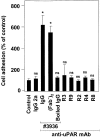
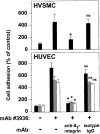
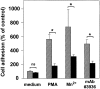
The urokinase receptor (CD87; uPAR) is found in close association with beta 2 integrins on leukocytes. We studied the functional consequence of this association for leukocyte adhesion and migration. In vivo, the beta 2 integrin-dependent recruitment of leukocytes to the inflamed peritoneum of uPAR-deficient mice was significantly reduced as compared with wild-type animals. In vitro, beta 2 integrin-mediated adhesion of leukocytes to endothelium was lost upon removal of uPAR from the leukocyte surface by phosphatidyl-inositol-specific phospholipase C. Leukocyte adhesion was reconstituted when soluble intact uPAR, but not a truncated form lacking the uPA-binding domain, was allowed to reassociate with the cell surface. uPAR ligation with a monoclonal antibody induced adhesion of monocytic cells and neutrophils to vascular endothelium by six- to eightfold, whereas ligation with inactivated uPA significantly reduced cell-to-cell adhesion irrespective of the beta 2 integrin-stimulating pathway. These data indicate that beta 2 integrin-mediated leukocyte-endothelial cell interactions and recruitment to inflamed areas require the presence of uPAR and define a new phenotype for uPAR-deficient mice. Moreover, uPAR ligation differentially modulates leukocyte adhesion to endothelium and provides novel targets for therapeutic strategies in inflammation-related vascular pathologies.
Figures







References
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. - PubMed
-
- Larson RS, Springer TA. Structure and function of leukocyte integrins. Immunol Rev. 1990;114:181–217. - PubMed
-
- Van der Vieren M, Le Trong H, Wood CL, Moore PF, St. John T, Staunton DE, Gallatin WM. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

