Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells
- PMID: 9743530
- PMCID: PMC2212541
- DOI: 10.1084/jem.188.6.1117
Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells
Abstract
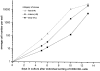
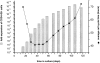
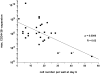
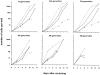
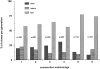
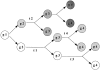
Hematopoietic stem cells (HSCs) in adult marrow are believed to be derived from fetal liver precursors. To study cell kinetics involved in long-term hematopoiesis, we studied single-sorted candidate HSCs from fetal liver that were cultured in the presence of a mixture of stimulatory cytokines. After 8-10 d, the number of cells in primary cultures varied from <100 to >10,000 cells. Single cells in slow growing colonies were recloned upon reaching a 100-200 cell stage. Strikingly, the number of cells in subclones varied widely again. These results are indicative of asymmetric divisions in primitive hematopoietic cells in which proliferative potential and cell cycle properties are unevenly distributed among daughter cells. The continuous generation of functional heterogeneity among the clonal progeny of HSCs is in support of intrinsic control of stem cell fate and provides a model for the long-term maintenance of hematopoiesis in vitro and in vivo.
Figures



References
-
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. - PubMed
-
- Metcalf, D. 1977. Hemopoietic Colonies. In Vitro Cloning of Normal and Leukemic Cells. Springer-Verlag, Berlin/ Heidelberg, Germany. 227 pp. - PubMed
-
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. - PubMed
-
- Lansdorp PM. Self-renewal of stem cells. Biol Blood Marrow Transplant. 1997;3:171–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

