Liver damage preferentially results from CD8(+) T cells triggered by high affinity peptide antigens
- PMID: 9743533
- PMCID: PMC2212548
- DOI: 10.1084/jem.188.6.1147
Liver damage preferentially results from CD8(+) T cells triggered by high affinity peptide antigens
Abstract
Little is understood of the anatomical fate of activated T lymphocytes and the consequences they have on the tissues into which they migrate. Previous work has suggested that damaged lymphocytes migrate to the liver. This study compares class I versus class II major histocompatibility complex (MHC)-restricted ovalbumin-specific T cell antigen receptor (TCR) transgenic mice to demonstrate that after in vivo activation with antigen the emergence of CD4(-)CD8(-)B220(+) T cells occurs more frequently from a CD8(+) precursor than from CD4(+) T cells. Furthermore, this change in phenotype is conferred only by the high affinity native peptide antigen and not by lower affinity peptide variants. After activation of CD8(+) cells with only the high affinity peptide, there is also a dramatically increased number of liver lymphocytes with accompanying extensive hepatocyte damage and elevation of serum aspartate transaminase. This was not observed in mice bearing a class II MHC-restricted TCR. The findings show that CD4(-)CD8(-)B220(+) T cells preferentially derive from a CD8(+) precursor after a high intensity TCR signal. After activation, T cells can migrate to the liver and induce hepatocyte damage, and thereby serve as a model of autoimmune hepatitis.
Figures




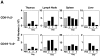
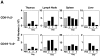





References
-
- Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. - PubMed
-
- Ju ST, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:345–347. - PubMed
-
- Huang L, Soldeville G, Leeker M, Flavell R, Crispe N. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

