Temperature effects on the allosteric responses of native and chimeric aspartate transcarbamoylases
- PMID: 9743634
- PMCID: PMC3233763
- DOI: 10.1006/jmbi.1998.2054
Temperature effects on the allosteric responses of native and chimeric aspartate transcarbamoylases
Abstract
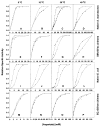
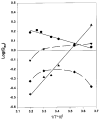
Although structurally very similar, the aspartate transcarbamoylases (ATCase) of Serratia marcescens and Escherichia coli have distinct allosteric regulatory patterns. It has been reported that a S. marcescens chimera, SM : rS5'ec, in which five divergent residues (r93 to r97) of the regulatory polypeptide were replaced with their Escherichia coli counterparts, possessed E. coli-like regulatory characteristics. The reverse chimera EC:rS5'sm, in which the same five residues of E. coli have been replaced with their S. marcescens counterpart, lost both heterotrophic and homotropic responses. These results indicate that the r93-r97 region is critical in defining the ATCase allosteric character. Molecular modeling of the regulatory polypeptides has suggested that the replacement of the S5' beta-strand resulted in disruption of the allosteric-zinc interface. However, the structure-function relationship could be indirect, and the disruption of the interface could influence allostery by altering the global energy of the enzyme. Studies of the temperature-sensitivity of the CTP response demonstrate that it is possible to convert CTP inhibition of the SM:rS5'ec chimera at high temperature to activation below 10 degreesC. Nonetheless, the temperature response of the native S. marcescens ATCase suggests a strong entropic effect that counteracts the CTP activation. Therefore, it is suggested that the entropy component of the coupling free energy plays a significant role in the determination of both the nature and magnitude of the allosteric effect in ATCase.
Copyright 1998 Academic Press.
Figures

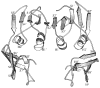


References
-
- Allewell NN, LiCata VJ. Thermodynamic approaches to understanding aspartate transcarbamylase. Methods Enzymol. 1995;259:608–628. - PubMed
-
- Aucoin JM, Pishko EJ, Baker DP, Kantrowitz ER. Engineered complementation in Escherichia coli aspartate transcarbamoylase-heterotropic regulation by quaternary structure stabilization. J Biol Chem. 1996;271:29865–29869. - PubMed
-
- Beck DA, Kedzie KM, Wild JR. Comparison of the aspartate transcarbamoylases from Serratia marcescens and Escherichia coli. J Biol Chem. 1989;264:16629–16637. - PubMed
-
- Braxton BL, Tlapak-Simmons VL, Reinhart GD. Temperature-induced inversion of allosteric phenomena. J Biol Chem. 1994;269:47–50. - PubMed
-
- Braxton BL, Mullins LS, Raushel FM, Reinhart GD. Allosteric effects of carbamoyl phosphate synthetase from Escherichia coli are entropy-driven. Biochemistry. 1996;35:11918–11924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

