Annexin XIIIb associates with lipid microdomains to function in apical delivery
- PMID: 9744874
- PMCID: PMC2141766
- DOI: 10.1083/jcb.142.6.1413
Annexin XIIIb associates with lipid microdomains to function in apical delivery
Abstract
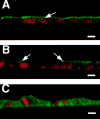
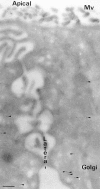
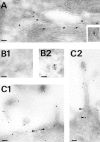
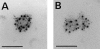
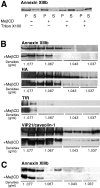
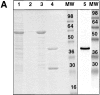
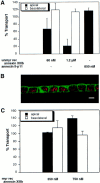
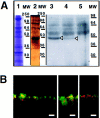
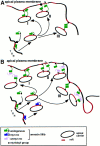
A member of the annexin XIII sub-family, annexin XIIIb, has been implicated in the apical exocytosis of epithelial kidney cells. Annexins are phospholipid-binding proteins that have been suggested to be involved in membrane trafficking events although their actual physiological function remains open. Unlike the other annexins, annexin XIIIs are myristoylated. Here, we show by immunoelectron microscopy that annexin XIIIb is localized to the trans-Golgi network (TGN), vesicular carriers and the apical cell surface. Polarized apical sorting involves clustering of apical proteins into dynamic sphingolipid-cholesterol rafts. We now provide evidence for the raft association of annexin XIIIb. Using in vitro assays and either myristoylated or unmyristoylated recombinant annexin XIIIb, we demonstrate that annexin XIIIb in its native myristoylated form stimulates specifically apical transport whereas the unmyristoylated form inhibits this route. Moreover, we show that formation of apical carriers from the TGN is inhibited by an anti-annexin XIIIb antibody whereas it is stimulated by myristoylated recombinant annexin XIIIb. These results suggest that annexin XIIIb directly participates in apical delivery.
Figures



References
-
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid/ cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model. Biochemistry. 1997;36:10944–10953. - PubMed
-
- Ali SM, Geisow MJ, Burgoyne RD. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989;340:313–315. - PubMed
-
- Beckers CJM, Keller DS, Balch WE. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the golgi complex. Cell. 1987;50:523–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

