Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice
- PMID: 9744877
- PMCID: PMC2141773
- DOI: 10.1083/jcb.142.6.1461
Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice
Abstract
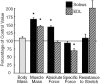
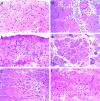
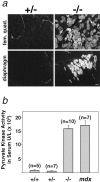
Limb-girdle muscular dystrophy type 2D (LGMD 2D) is an autosomal recessive disorder caused by mutations in the alpha-sarcoglycan gene. To determine how alpha-sarcoglycan deficiency leads to muscle fiber degeneration, we generated and analyzed alpha-sarcoglycan- deficient mice. Sgca-null mice developed progressive muscular dystrophy and, in contrast to other animal models for muscular dystrophy, showed ongoing muscle necrosis with age, a hallmark of the human disease. Sgca-null mice also revealed loss of sarcolemmal integrity, elevated serum levels of muscle enzymes, increased muscle masses, and changes in the generation of absolute force. Molecular analysis of Sgca-null mice demonstrated that the absence of alpha-sarcoglycan resulted in the complete loss of the sarcoglycan complex, sarcospan, and a disruption of alpha-dystroglycan association with membranes. In contrast, no change in the expression of epsilon-sarcoglycan (alpha-sarcoglycan homologue) was observed. Recombinant alpha-sarcoglycan adenovirus injection into Sgca-deficient muscles restored the sarcoglycan complex and sarcospan to the membrane. We propose that the sarcoglycan-sarcospan complex is requisite for stable association of alpha-dystroglycan with the sarcolemma. The Sgca-deficient mice will be a valuable model for elucidating the pathogenesis of sarcoglycan deficient limb-girdle muscular dystrophies and for the development of therapeutic strategies for this disease.
Figures





References
-
- Allamand V, Sunada Y, Salih MA, Straub V, Ozo CO, Al-Turaiki MH, Akbar M, Kolo T, Colognato H, Zhang X, Sorokin LM, Yurchenco PD, Tryggvason K, Campbell KP. Mild congenital muscular dystrophy in two patients with an internally deleted laminin alpha2-chain. Hum Mol Genet. 1997;6:747–752. - PubMed
-
- Bönnemann CG, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E, McNally EM, Duggan DJ, Angelini C, et al. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet. 1995;11:266–273. - PubMed
-
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. - PubMed
-
- Carrie A, Piccolo F, Leturcq F, de Toma C, Azibi K, Beldjord C, Vallat JM, Merlini L, Voit T, Sewry C, Urtizberea JA, Romero N, Tome FM, Fardeau M, Sunada Y, Campbell KP, Kaplan JC, Jeanpierre M. Mutational diversity and hot spots in the alpha-sarcoglycan gene in autosomal recessive muscular dystrophy (LGMD2D) J Med Genet. 1997;34:470–475. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

