The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity
- PMID: 9744888
- PMCID: PMC2141769
- DOI: 10.1083/jcb.142.6.1605
The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity
Abstract
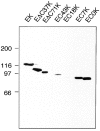
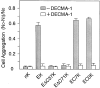
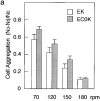
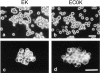
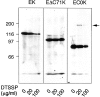
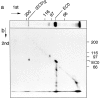
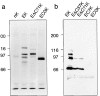
Cadherins are transmembrane glycoproteins involved in Ca2+-dependent cell-cell adhesion. Deletion of the COOH-terminal residues of the E-cadherin cytoplasmic domain has been shown to abolish its cell adhesive activity, which has been ascribed to the failure of the deletion mutants to associate with catenins. Based on our present results, this concept needs revision. As was reported previously, leukemia cells (K562) expressing E-cadherin with COOH-terminal deletion of 37 or 71 amino acid residues showed almost no aggregation. Cells expressing E-cadherin with further deletion of 144 or 151 amino acid residues, which eliminates the membrane-proximal region of the cytoplasmic domain, showed E-cadherin-dependent aggregation. Thus, deletion of the membrane-proximal region results in activation of the nonfunctional E-cadherin polypeptides. However, these cells did not show compaction. Chemical cross-linking revealed that the activated E-cadherin polypeptides can be cross-linked to a dimer on the surface of cells, whereas the inactive polypeptides, as well as the wild-type E-cadherin polypeptide containing the membrane-proximal region, can not. Therefore, the membrane-proximal region participates in regulation of the adhesive activity by preventing lateral dimerization of the extracellular domain.
Figures


Similar articles
-
p120-independent modulation of E-cadherin adhesion activity by the membrane-proximal region of the cytoplasmic domain.J Biol Chem. 2003 Nov 14;278(46):46014-20. doi: 10.1074/jbc.M307778200. Epub 2003 Sep 2. J Biol Chem. 2003. PMID: 12952959
-
p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity.J Biol Chem. 1999 Jul 23;274(30):21409-15. doi: 10.1074/jbc.274.30.21409. J Biol Chem. 1999. PMID: 10409703
-
The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn.J Cell Biol. 1998 May 4;141(3):779-89. doi: 10.1083/jcb.141.3.779. J Cell Biol. 1998. PMID: 9566976 Free PMC article.
-
Role of p120-catenin in cadherin trafficking.Biochim Biophys Acta. 2007 Jan;1773(1):8-16. doi: 10.1016/j.bbamcr.2006.07.005. Epub 2006 Jul 21. Biochim Biophys Acta. 2007. PMID: 16949165 Review.
-
Controversies at the cytoplasmic face of the cadherin-based adhesion complex.Curr Opin Cell Biol. 1999 Oct;11(5):567-72. doi: 10.1016/s0955-0674(99)00015-0. Curr Opin Cell Biol. 1999. PMID: 10508647 Review.
Cited by
-
Cadherins mediate both the association between PS1 and beta-catenin and the effects of PS1 on beta-catenin stability.J Biol Chem. 2005 Oct 28;280(43):36007-12. doi: 10.1074/jbc.M507503200. Epub 2005 Aug 26. J Biol Chem. 2005. PMID: 16126725 Free PMC article.
-
Crystal structure of the M-fragment of alpha-catenin: implications for modulation of cell adhesion.EMBO J. 2001 Jul 16;20(14):3645-56. doi: 10.1093/emboj/20.14.3645. EMBO J. 2001. PMID: 11447106 Free PMC article.
-
Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion.J Cell Biol. 2000 Jan 10;148(1):189-202. doi: 10.1083/jcb.148.1.189. J Cell Biol. 2000. PMID: 10629228 Free PMC article.
-
Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component.J Cell Biol. 2003 Feb 3;160(3):433-49. doi: 10.1083/jcb.200211083. Epub 2003 Jan 27. J Cell Biol. 2003. PMID: 12551951 Free PMC article.
-
SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation.Dev Biol. 2010 Aug 15;344(2):731-44. doi: 10.1016/j.ydbio.2010.05.507. Epub 2010 May 31. Dev Biol. 2010. PMID: 20515680 Free PMC article.
References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

