Surface-associated hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model
- PMID: 9746556
- PMCID: PMC108567
- DOI: 10.1128/IAI.66.10.4602-4610.1998
Surface-associated hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model
Abstract
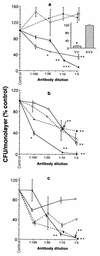
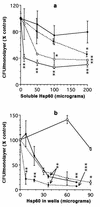
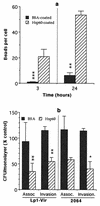
HeLa cells have been previously used to demonstrate that virulent strains of Legionella pneumophila (but not salt-tolerant avirulent strains) efficiently invade nonphagocytic cells. Hsp60, a member of the GroEL family of chaperonins, is displayed on the surface of virulent L. pneumophila (R. A. Garduño et al., J. Bacteriol. 180:505-513, 1988). Because Hsp60 is largely involved in protein-protein interactions, we investigated its role in adherence-invasion in the HeLa cell model. Hsp60-specific antibodies inhibited the adherence and invasiveness of two virulent L. pneumophila strains in a dose-dependent manner but had no effect on the association of their salt-tolerant avirulent derivatives with HeLa cells. A monospecific anti-OmpS (major outer membrane protein) serum inhibited the association of both virulent and avirulent strains of L. pneumophila to HeLa cells, suggesting that while both Hsp60 and OmpS may mediate bacterial association to HeLa cells, only virulent strains selectively displayed Hsp60 on their surfaces. Furthermore, the surface-associated Hsp60 of virulent bacterial cells was susceptible to the action of trypsin, which rendered the bacteria noninvasive. Additionally, pretreatment of HeLa cells with purified Hsp60 or precoating of the plastic surface where HeLa cells attached with Hsp60 reduced the adherence and invasiveness of the two virulent strains. Finally, recombinant Hsp60 covalently bound to latex beads promoted the early association of beads with HeLa cells by a factor of 20 over bovine serum albumin (BSA)-coated beads and competed with virulent strains for association with HeLa cells. Hsp60-coated beads were internalized in large numbers by HeLa cells and remained in tight endosomes that did not fuse with other vesicles, whereas internalized BSA-coated beads, for which endocytic trafficking is well established, resided in more loose or elongated endosomes. Mature intracellular forms of L. pneumophila, which were up to 100-fold more efficient than agar-grown bacteria at associating with HeLa cells, were enriched for Hsp60 on the bacterial surface, as determined by immunolocalization techniques. Collectively, these results establish a role for surface-exposed Hsp60 in invasion of HeLa cells by L. pneumophila.
Figures



References
-
- Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

