Filament tip-associated antigens involved in adherence to and invasion of murine pulmonary epithelial cells in vivo and HeLa cells in vitro by Nocardia asteroides
- PMID: 9746564
- PMCID: PMC108575
- DOI: 10.1128/IAI.66.10.4676-4689.1998
Filament tip-associated antigens involved in adherence to and invasion of murine pulmonary epithelial cells in vivo and HeLa cells in vitro by Nocardia asteroides
Abstract
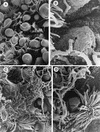
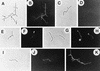
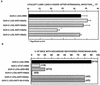
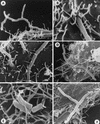
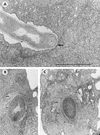
The interactions of Nocardia asteroides GUH-2 with pulmonary epithelial cells of C57BL/6 mice and with HeLa cells were studied. Electron microscopy demonstrated that only the tips of log-phase cells penetrated pulmonary epithelial cells following intranasal administration, and nocardiae were recovered from the brain. Coccobacillary cells neither invaded nor disseminated. Serum from immunized mice (IMS) decreased attachment to and penetration of pulmonary epithelial cell surfaces by log-phase GUH-2 and inhibited spread to the brain. IMS was adsorbed against stationary-phase cells. Western immunoblots suggested that this adsorbed IMS was reactive primarily with 43- and 62-kDa proteins. Immunofluorescence showed that adsorbed IMS preferentially labeled the tips of log-phase GUH-2 cells. Since this IMS was reactive to culture filtrate antigens, several of these proteins were cut from gels, and mice were immunized. Sera against 62-, 55-, 43-, 36-, 31-, and 25-kDa antigens were obtained. The antisera against the 43- and 36-kDa proteins labeled the filament tips of GUH-2 cells. Only the antiserum against the 43-kDa antigen increased pulmonary clearance, inhibited apical attachment to and penetration of pulmonary epithelial cells, and prevented spread to the brain. An in vitro model with HeLa cells demonstrated that the tips of log-phase cells of GUH-2 adhered to and penetrated the surface of HeLa cells. Invasion assays with amikacin treatment demonstrated that nocardiae were internalized. Adsorbed IMS blocked attachment to and invasion of these cells. These data suggested that a filament tip-associated 43-kDa protein was involved in attachment to and invasion of pulmonary epithelial cells and HeLa cells by N. asteroides GUH-2.
Figures





References
-
- Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley L W. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. Science. 1993;261:1454–1456. - PubMed
-
- Baba T, Nishiuchi Y, Yano I. Composition of mycolic acid molecular species as a criterion in nocardial classification. Int J Syst Bacteriol. 1997;47:795–801.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

