Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development
- PMID: 9746587
- PMCID: PMC108598
- DOI: 10.1128/IAI.66.10.4838-4844.1998
Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development
Abstract
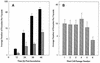
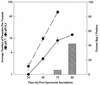
Tachyzoites (VEG strain) that emerge from host cells infected with Toxoplasma gondii sporozoites proliferate relatively fast and double their number every 6 h. This rate of growth is intrinsic, as neither the number of host cells invaded nor host cell type appears to influence emergent tachyzoite replication. Fast tachyzoite growth was not persistent, and following approximately 20 divisions, the population uniformly shifted to slower growth. Parasites 10 days post-sporozoite infection doubled only once every 15 h and, unlike emergent tachyzoites, they grew at this slower rate over several months of continuous cell culture. The spontaneous change in tachyzoite growth rate preceded the expression of the bradyzoite-specific marker, BAG1. Within 24 h of the growth shift, 2% of the population expressed BAG1, and by 15 days post-sporozoite infection, 50% of the parasites were positive for this marker. Spontaneous BAG1 expression was not observed in sporozoites or in tachyzoites during fast growth (through day 6 post-sporozoite inoculation), although these tachyzoites could be induced to express BAG1 earlier by culturing sporozoite-infected cells at pH 8.3. However, alkaline treatment also reduced the replication of emergent tachyzoites to the rate of growth-shifted parasites, supporting a link between reduced parasite growth and bradyzoite differentiation. The shift to slower growth was closely correlated with virulence in mice, as the initially fast-growing emergent tachyzoites were avirulent (100% lethal dose, >10(4) parasites), while a mutant VEG strain (MS-J) that is unable to growth shift caused 100% mortality in mice inoculated with 10 parasites. Parasites recovered from gamma interferon knockout mice inoculated with emergent tachyzoites grew at a slow rate and expressed BAG1, confirming that the replication switch occurs in animals and in the absence of a protective immune response.
Figures





Similar articles
-
Real-time RT-PCR on SAG1 and BAG1 gene expression during stage conversion in immunosuppressed mice infected with Toxoplasma gondii Tehran strain.Korean J Parasitol. 2012 Sep;50(3):199-205. doi: 10.3347/kjp.2012.50.3.199. Epub 2012 Aug 13. Korean J Parasitol. 2012. PMID: 22949746 Free PMC article.
-
Unexpected oocyst shedding by cats fed Toxoplasma gondii tachyzoites: in vivo stage conversion and strain variation.Vet Parasitol. 2005 Nov 5;133(4):289-98. doi: 10.1016/j.vetpar.2005.06.007. Vet Parasitol. 2005. PMID: 16024176
-
Targeted disruption of the bradyzoite-specific gene BAG1 does not prevent tissue cyst formation in Toxoplasma gondii.Mol Biochem Parasitol. 1998 May 1;92(2):291-301. doi: 10.1016/s0166-6851(97)00236-3. Mol Biochem Parasitol. 1998. PMID: 9657333
-
Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts.Clin Microbiol Rev. 1998 Apr;11(2):267-99. doi: 10.1128/CMR.11.2.267. Clin Microbiol Rev. 1998. PMID: 9564564 Free PMC article. Review.
-
Stress-related and spontaneous stage differentiation of Toxoplasma gondii.Mol Biosyst. 2008 Aug;4(8):824-34. doi: 10.1039/b800520f. Epub 2008 Jun 2. Mol Biosyst. 2008. PMID: 18633484 Review.
Cited by
-
Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development.PLoS Pathog. 2006 Oct;2(10):e105. doi: 10.1371/journal.ppat.0020105. PLoS Pathog. 2006. PMID: 17069459 Free PMC article.
-
Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis.PLoS Pathog. 2018 May 2;14(5):e1007035. doi: 10.1371/journal.ppat.1007035. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29718996 Free PMC article.
-
Restriction Checkpoint Controls Bradyzoite Development in Toxoplasma gondii.Microbiol Spectr. 2022 Jun 29;10(3):e0070222. doi: 10.1128/spectrum.00702-22. Epub 2022 Jun 2. Microbiol Spectr. 2022. PMID: 35652638 Free PMC article.
-
ROP16-mediated activation of STAT6 enhances cyst development of type III Toxoplasma gondii in neurons.PLoS Pathog. 2023 Apr 17;19(4):e1011347. doi: 10.1371/journal.ppat.1011347. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37068104 Free PMC article.
-
Generation of Toxoplasma gondii and Hammondia hammondi Oocysts and Purification of Their Sporozoites for Downstream Manipulation.Methods Mol Biol. 2020;2071:81-98. doi: 10.1007/978-1-4939-9857-9_4. Methods Mol Biol. 2020. PMID: 31758447 Free PMC article.
References
-
- Bohne W, Gross U, Ferguson D J P, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. - PubMed
-
- Burg J L, Perelman D, Kaspar L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. - PubMed
-
- Dalton D K, Pitts M S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

