Murine dendritic cells pulsed in vitro with Toxoplasma gondii antigens induce protective immunity in vivo
- PMID: 9746591
- PMCID: PMC108602
- DOI: 10.1128/IAI.66.10.4867-4874.1998
Murine dendritic cells pulsed in vitro with Toxoplasma gondii antigens induce protective immunity in vivo
Abstract
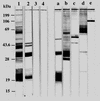
The activation of a predominant T-helper-cell subset plays a critical role in disease resolution. In the case of Toxoplasma gondii, the available evidence indicates that CD4(+) protective cells belong to the Th1 subset. The aim of this study was to determine whether T. gondii antigens (in T. gondii sonicate [TSo]) presented by splenic dendritic cells (DC) were able to induce a specific immune response in vivo and to protect CBA/J mice orally challenged with T. gondii cysts. CBA/J mice immunized with TSo-pulsed DC exhibited significantly fewer cysts in their brains after oral infection with T. gondii 76K than control mice did. Protected mice developed a strong humoral response in vivo, with especially high levels of anti-TSo immunoglobulin G2a antibodies in serum. T. gondii antigens such as SAG1 (surface protein), SAG2 (surface protein), MIC1 (microneme protein), ROP2 through ROP4 (rhoptry proteins), and MIC2 (microneme protein) were recognized predominantly. Furthermore, DC loaded with TSo, which synthesized high levels of interleukin-12 (IL-12), triggered a strong cellular response in vivo, as assessed by the proliferation of lymph node cells in response to TSo restimulation in vitro. Cellular proliferation was associated with gamma interferon and IL-2 production. Taken together, these results indicate that immunization of CBA/J mice with TSo-pulsed DC can induce both humoral and Th1-like cellular immune responses and affords partial resistance against the establishment of chronic toxoplasmosis.
Figures
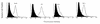



References
-
- Barratt-Boyes S M, Watkins S C, Finn O J. In vivo migration of dendritic cells differentiated in vitro. A chimpanzee model. J Immunol. 1997;158:4543–4547. - PubMed
-
- Bluestone J A. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. - PubMed
-
- Bülow R, Boothroyd J C. Protection of mice from fatal Toxoplasma gondii infection by immunization with P30 antigen in liposomes. J Immunol. 1991;147:3496–3500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

