Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms
- PMID: 9746592
- PMCID: PMC108603
- DOI: 10.1128/IAI.66.10.4875-4883.1998
Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms
Abstract
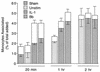
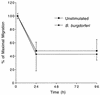
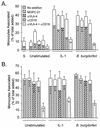
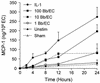
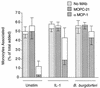
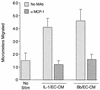
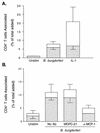
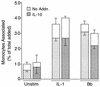
A prominent feature of Lyme disease is the perivascular accumulation of mononuclear leukocytes. Incubation of human umbilical vein endothelial cells (HUVEC) cultured on amniotic tissue with either interleukin-1 (IL-1) or Borrelia burgdorferi, the spirochetal agent of Lyme disease, increased the rate at which human monocytes migrated across the endothelial monolayers. Very late antigen 4 (VLA-4) and CD11/CD18 integrins mediated migration of monocytes across HUVEC exposed to either B. burgdorferi or IL-1 in similar manners. Neutralizing antibodies to the chemokine monocyte chemoattractant protein 1 (MCP-1) inhibited the migration of monocytes across unstimulated, IL-1-treated, or B. burgdorferi-stimulated HUVEC by 91% +/- 3%, 65% +/- 2%, or 25% +/- 22%, respectively. Stimulation of HUVEC with B. burgdorferi also promoted a 6-fold +/- 2-fold increase in the migration of human CD4(+) T lymphocytes. Although MCP-1 played only a limited role in the migration of monocytes across B. burgdorferi-treated HUVEC, migration of CD4(+) T lymphocytes across HUVEC exposed to spirochetes was highly dependent on this chemokine. The anti-inflammatory cytokine IL-10 reduced both migration of monocytes and endothelial production of MCP-1 in response to B. burgdorferi by approximately 50%, yet IL-10 inhibited neither migration nor secretion of MCP-1 when HUVEC were stimulated with IL-1. Our results suggest that activation of endothelium by B. burgdorferi may contribute to formation of the chronic inflammatory infiltrates associated with Lyme disease. The transendothelial migration of monocytes that is induced by B. burgdorferi is significantly less dependent on MCP-1 than is migration induced by IL-1. Selective inhibition by IL-10 further indicates that B. burgdorferi and IL-1 employ distinct mechanisms to activate endothelial cells.
Figures
References
-
- Anonymous. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. - PubMed
-
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
-
- Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
-
- Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

