Surface structure, hydrophobicity, phagocytosis, and adherence to matrix proteins of Bacillus cereus cells with and without the crystalline surface protein layer
- PMID: 9746594
- PMCID: PMC108605
- DOI: 10.1128/IAI.66.10.4895-4902.1998
Surface structure, hydrophobicity, phagocytosis, and adherence to matrix proteins of Bacillus cereus cells with and without the crystalline surface protein layer
Abstract
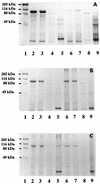
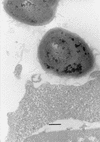
Nonopsonic phagocytosis of Bacillus cereus by human polymorphonuclear leukocytes (PMNs) with particular attention to bacterial surface properties and structure was studied. Two reference strains (ATCC 14579(T) and ATCC 4342) and two clinical isolates (OH599 and OH600) from periodontal and endodontic infections were assessed for adherence to matrix proteins, such as type I collagen, fibronectin, laminin, and fibrinogen. One-day-old cultures of strains OH599 and OH600 were readily ingested by PMNs in the absence of opsonins, while cells from 6-day-old cultures were resistant. Both young and old cultures of the reference strains of B. cereus were resistant to PMN ingestion. Preincubation of PMNs with the phagocytosis-resistant strains of B. cereus did not affect the phagocytosis of the sensitive strain. Negatively stained cells of OH599 and OH600 studied by electron microscopy had a crystalline protein layer on the cell surface. In thin-sectioned cells of older cultures (3 to 6 days old), the S-layer was observed to peel off from the cells. No S-layer was detected on the reference strains. Extraction of cells with detergent followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a major 97-kDa protein from the strains OH599 and OH600 but only a weak 97-kDa band from the reference strain ATCC 4342. One-day-old cultures of the clinical strains (hydrophobicity, 5.9 to 6.0%) showed strong binding to type I collagen, laminin, and fibronectin. In contrast, reference strains (hydrophobicity, -1.0 to 4.2%) as well as 6-day-old cultures of clinical strains (hydrophobicity, 19.0 to 53.0%) bound in only low numbers to the proteins. Gold-labelled biotinylated fibronectin was localized on the S-layer on the cell surface as well as on fragments of S-layer peeling off the cells of a 6-day-old culture of B. cereus OH599. Lactose, fibronectin, laminin, and antibodies against the S-protein reduced binding to laminin but not to fibronectin. Heating the cells at 84 degreesC totally abolished binding to both proteins. Benzamidine, a noncompetitive serine protease inhibitor, strongly inhibited binding to fibronectin whereas binding to laminin was increased. Overall, the results indicate that changes in the surface structure, evidently involving the S-layer, during growth of the clinical strains of B. cereus cause a shift from susceptibility to PMN ingestion and strong binding to matrix and basement membrane proteins. Furthermore, it seems that binding to laminin is mediated by the S-protein while binding to fibronectin is dependent on active protease evidently attached to the S-layer.
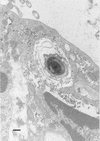
Figures




References
-
- Absolom D R. The role of bacterial hydrophobicity in infection: bacterial adhesion and phagocytic ingestion. Can J Microbiol. 1988;34:287–298. - PubMed
-
- Beveridge T J. The response of S-layered bacteria to the Gram stain. FEMS Microbiol Rev. 1997;20:101–110.
-
- Blaser M J. Role of the S-layer proteins of Campylobacter fetus in serum-resistance and antigenic variation: a model of bacterial pathogenesis. Am J Med Sci. 1993;306:325–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

