Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents
- PMID: 9746610
- PMCID: PMC108621
- DOI: 10.1128/IAI.66.10.5001-5007.1998
Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents
Abstract
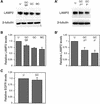
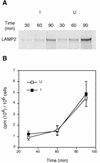
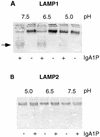
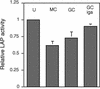
Members of our group reported recently that neisseria infection of human epithelial cells results in accelerated degradation of the major lysosomal integral membrane protein LAMP1 and that this is due to hydrolysis of this glycoprotein at its immunoglobulin A1 (IgA1)-like hinge by the neisseria type 2 IgA1 protease (L. Lin et al., Mol. Microbiol. 24:1083-1094, 1997). We also reported that the IgA1 protease plays a major role in the ability of the pathogenic neisseriae to survive within epithelial cells and hypothesized that this is due to alteration of lysosomes as a result of protease-mediated LAMP1 degradation. In this study, we tested the hypothesis that neisseria infection leads to multiple changes in lysosomes. Here, we report that neisseria infection also reduces the levels of three other lysosomal markers: LAMP2, lysosomal acid phosphatase (LAP), and CD63. In contrast, neither the epidermal growth factor receptor level nor the beta-tubulin level is affected. A detailed examination of LAMP2 indicated that the reduced LAMP2 levels are not the result of an altered biosynthetic rate or of cleavage by the IgA1 protease. Nevertheless, the protease plays a role in reducing LAMP2 and LAP activity levels, as these are partially restored in cells infected with an iga mutant. We conclude that neisseria infection results in multiple changes to the lysosomes of infected epithelial cells and that these changes are likely an indirect result of IgA1 protease-mediated cleavage of LAMP1.
Figures


Similar articles
-
The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells.Mol Microbiol. 1997 Jun;24(5):1083-94. doi: 10.1046/j.1365-2958.1997.4191776.x. Mol Microbiol. 1997. PMID: 9220014
-
The pilus-induced Ca2+ flux triggers lysosome exocytosis and increases the amount of Lamp1 accessible to Neisseria IgA1 protease.Cell Microbiol. 2001 Apr;3(4):265-75. doi: 10.1046/j.1462-5822.2001.00112.x. Cell Microbiol. 2001. PMID: 11298650
-
Effects of the immunoglobulin A1 protease on Neisseria gonorrhoeae trafficking across polarized T84 epithelial monolayers.Infect Immun. 2000 Feb;68(2):906-11. doi: 10.1128/IAI.68.2.906-911.2000. Infect Immun. 2000. PMID: 10639461 Free PMC article.
-
'Small' talk: Opa proteins as mediators of Neisseria-host-cell communication.Curr Opin Microbiol. 2003 Feb;6(1):43-9. doi: 10.1016/s1369-5274(03)00004-3. Curr Opin Microbiol. 2003. PMID: 12615218 Review.
-
Proteases of the pathogenic neisseriae: possible role in infection.Microbios. 1986;45(183):113-29. Microbios. 1986. PMID: 3086672 Review.
Cited by
-
Update on meningococcal disease with emphasis on pathogenesis and clinical management.Clin Microbiol Rev. 2000 Jan;13(1):144-66, table of contents. doi: 10.1128/CMR.13.1.144. Clin Microbiol Rev. 2000. PMID: 10627495 Free PMC article. Review.
-
Active-site gating regulates substrate selectivity in a chymotrypsin-like serine protease the structure of haemophilus influenzae immunoglobulin A1 protease.J Mol Biol. 2009 Jun 12;389(3):559-74. doi: 10.1016/j.jmb.2009.04.041. Epub 2009 Apr 23. J Mol Biol. 2009. PMID: 19393662 Free PMC article.
-
Characterisation of Aspergillus fumigatus Endocytic Trafficking within Airway Epithelial Cells Using High-Resolution Automated Quantitative Confocal Microscopy.J Fungi (Basel). 2021 Jun 7;7(6):454. doi: 10.3390/jof7060454. J Fungi (Basel). 2021. PMID: 34200399 Free PMC article.
-
Meningococcal pneumonia: a review.Pneumonia (Nathan). 2019 Aug 25;11:3. doi: 10.1186/s41479-019-0062-0. eCollection 2019. Pneumonia (Nathan). 2019. PMID: 31463180 Free PMC article. Review.
-
Pilus-mediated co-aggregation with Lactobacillus crispatus increases meningococcal susceptibility to antimicrobial agents by interfering with microcolony formation.BMC Microbiol. 2025 Jul 30;25(1):467. doi: 10.1186/s12866-025-04201-2. BMC Microbiol. 2025. PMID: 40739165 Free PMC article.
References
-
- Barriocanal J G, Bonifacino J S, Yuan L, Sandoval I V. Biosynthesis, glycosylation, movement through the Golgi system and transport to lysosomes by an N-linked carbohydrate independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986;261:16755–16763. - PubMed
-
- Carlsson S R, Roth J, Piller F, Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp1 and h-lamp2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988;263:18911–18919. - PubMed
-
- Cha Y, Holland S M, August J T. The cDNA sequence of mouse LAMP-2. J Biol Chem. 1990;265:5008–5013. - PubMed
-
- Chen J W, Chen G L, D’Souza M P, Murphy T L, August J T. Lysosomal membrane glycoproteins: properties of LAMP-1 and LAMP-2. Biochem Soc Symp. 1986;51:97–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

