Real time detection of DNA.RNA hybridization in living cells
- PMID: 9751701
- PMCID: PMC21676
- DOI: 10.1073/pnas.95.20.11538
Real time detection of DNA.RNA hybridization in living cells
Abstract
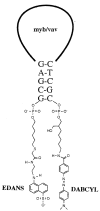
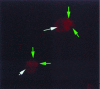
Demonstrating hybridization between an antisense oligodeoxynucleotide and its mRNA target has proven to be extremely difficult in living cells. To address this fundamental problem in antisense research, we synthesized "molecular beacon" (MB) reporter oligodeoxynucleotides with matched fluorescent donor and acceptor chromophores on their 5' and 3' ends. In the absence of a complementary nucleic acid strand, the MB remains in a stem-loop conformation where fluorescence resonance energy transfer prevents signal emission. On hybridization with a complementary sequence, the stem-loop structure opens increasing the physical distance between the donor and acceptor moieties thereby reducing fluorescence resonance energy transfer and allowing a detectable signal to be emitted when the beacon is excited by light of the appropriate wavelength. Solution hybridization studies revealed that in the presence of a complementary strand targeted MB could yield up to a 60-fold increase in fluorescence intensity in comparison to control MB. By using a fluorescence microscope fitted with UV fluoride lenses, the detection limit of preformed MB/target sequence duplexes microinjected into cells was found to be >/=1 x 10(-1) ag of MB, or approximately 10 molecules of mRNA. On the basis of this exquisite sensitivity, real-time detection of MB/target mRNA hybridization in living cells was attempted by microinjecting MB targeted to the vav protooncogene, or control MB, into K562 human leukemia cells. Within 15 min, confocal microscopy revealed fluorescence in cells injected with targeted, but not control, MB. These studies suggest that real-time visualization and localization of oligonucleotide/mRNA interactions is now possible. MB could find utility in studying RNA processing, trafficking, and folding in living cells. We hypothesize that MB may also prove useful for finding targetable mRNA sequence under physiologic conditions.
Figures

References
-
- Leeds J M, Henry S P, Truong L, Zutshi A, Levin A A, Kornbrust D. Drug Metab Dispos. 1997;25:921–926. - PubMed
-
- Dzau V J, Morishita R, Gibbons G H. Trends Biotechnol. 1993;11:205–210. - PubMed
-
- Morishita R, Gibbons G H, Kaneda Y, Ogihara T, Dzau V J. Gene. 1994;149:13–19. - PubMed
-
- Bold R J, Warren R E, Ishizuka J, Cho-Chung Y S, Townsend C M, Jr, Thompson J C. Surgery. 1994;116:189–195. ; discussion 195–196. - PubMed
-
- Chang A G, Wu G Y. Gastroenterology. 1994;106:1076–1084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

