Apoptotic proteins Reaper and Grim induce stable inactivation in voltage-gated K+ channels
- PMID: 9751729
- PMCID: PMC21704
- DOI: 10.1073/pnas.95.20.11703
Apoptotic proteins Reaper and Grim induce stable inactivation in voltage-gated K+ channels
Abstract
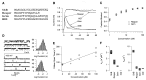
Drosophila genes reaper, grim, and head-involution-defective (hid) induce apoptosis in several cellular contexts. N-terminal sequences of these proteins are highly conserved and are similar to N-terminal inactivation domains of voltage-gated potassium (K+) channels. Synthetic Reaper and Grim N terminus peptides induced fast inactivation of Shaker-type K+ channels when applied to the cytoplasmic side of the channel that was qualitatively similar to the inactivation produced by other K+ channel inactivation particles. Mutations that reduce the apoptotic activity of Reaper also reduced the synthetic peptide's ability to induce channel inactivation, indicating that K+ channel inactivation correlated with apoptotic activity. Coexpression of Reaper RNA or direct injection of full length Reaper protein caused near irreversible block of the K+ channels. These results suggest that Reaper and Grim may participate in initiating apoptosis by stably blocking K+ channels.
Figures


References
-
- Ameisen J C. Science. 1996;272:1278–1279. - PubMed
-
- Thompson C B. Science. 1995;267:1456–1462. - PubMed
-
- Schwartz L M, Milligan C E. Trends Neurosci. 1996;19:555–562. - PubMed
-
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. Nature (London) 1998;391:43–50. - PubMed
-
- White K, Grether M E, Abrams J M, Young L, Farrell K, Steller H. Science. 1994;264:677–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

