The PEBP2betaMYH11 fusion created by Inv(16)(p13;q22) in myeloid leukemia impairs neutrophil maturation and contributes to granulocytic dysplasia
- PMID: 9751756
- PMCID: PMC21731
- DOI: 10.1073/pnas.95.20.11863
The PEBP2betaMYH11 fusion created by Inv(16)(p13;q22) in myeloid leukemia impairs neutrophil maturation and contributes to granulocytic dysplasia
Abstract
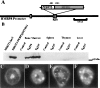
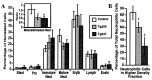
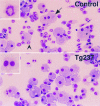
Chromosomal translocations involving the genes encoding the alpha and beta subunits of the Pebp2/Cbf transcription factor have been associated with human acute myeloid leukemia and the preleukemic condition, myelodysplasia. Inv(16)(p13;q22) fuses the gene encoding the beta subunit of Pebp2 to the MYH11 gene encoding a smooth muscle myosin heavy chain (Smmhc). To examine the effect of the inv(16)(p13;q22) on myelopoiesis, we used the hMRP8 promoter element to generate transgenic mice expressing the Pebp2betaSmmhc chimeric fusion protein in myeloid cells. Neutrophil maturation was impaired in PEBP2betaMYH11 transgenic mice. Although the transgenic mice had normal numbers of circulating neutrophils, their bone marrow contained increased numbers of immature neutrophilic cells, which exhibited abnormal characteristics. In addition, PEBP2betaMYH11 inhibited neutrophilic differentiation in colonies derived from hematopoietic progenitors. Coexpression of both PEBP2betaMYH11 and activated NRAS induced a more severe phenotype characterized by abnormal nuclear morphology indicative of granulocytic dysplasia. These results show that PEBP2betaMYH11 can impair neutrophil development and provide evidence that alterations of Pebp2 can contribute to the genesis of myelodysplasia.
Figures

References
-
- Pedersen-Bjergaard J, Rowley J D. Blood. 1994;83:2780–2786. - PubMed
-
- Liu P, Tarle S A, Hajra A, Claxton D F, Marlton P, Freedman M, Siciliano M J, Collins F S. Science. 1993;261:1041–1044. - PubMed
-
- Le Beau M M, Larson R A, Bitter M A, Vardiman J W, Golomb H M, Rowley J D. N Engl J Med. 1983;309:630–636. - PubMed
-
- Langabeer S E, Walker H, Gale R E, Wheatley K, Burnett A K, Goldstone A H, Linch D C. Br J Haematol. 1997;96:736–739. - PubMed
-
- Speck N A, Stacy T. In: Critical Reviews in Eukaryotic Gene Expression. Stein G S, Stein J L, Lian J B, editors. New York: Begell House; 1995. pp. 337–364.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

