Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D
- PMID: 9751757
- PMCID: PMC21732
- DOI: 10.1073/pnas.95.20.11869
Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D
Abstract
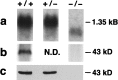
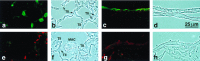
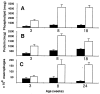
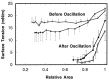
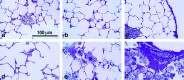
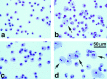
Surfactant protein D (SP-D) is one of two collectins found in the pulmonary alveolus. On the basis of homology with other collectins, potential functions for SP-D include roles in innate immunity and surfactant metabolism. The SP-D gene was disrupted in embryonic stem cells by homologous recombination to generate mice deficient in SP-D. Mice heterozygous for the mutant SP-D allele had SP-D concentrations that were approximately 50% wild type but no other obvious phenotypic abnormality. Mice totally deficient in SP-D were healthy to 7 months but had a progressive accumulation of surfactant lipids, SP-A, and SP-B in the alveolar space. By 8 weeks the alveolar phospholipid pool was 8-fold higher than wild-type littermates. There was also a 10-fold accumulation of alveolar macrophages in the null mice, and many macrophages were both multinucleated and foamy in appearance. Type II cells in the null mice were hyperplastic and contained giant lamellar bodies. These alterations in surfactant homeostasis were not associated with detectable changes in surfactant surface activity, postnatal respiratory function, or survival. The findings in the SP-D-deficient mice suggest a role for SP-D in surfactant homeostasis.
Figures


References
-
- Persson A, Chang D, Rust K, Moxley M, Longmore W, Crouch E. Biochemistry. 1989;28:6361–6367. - PubMed
-
- Wong C J, Akiyama J, Allen L, Hawgood S. Pediatr Res. 1996;39:930–937. - PubMed
-
- Fisher J H, Mason R. Am J Respir Cell Mol Biol. 1995;12:13–18. - PubMed
-
- Akiyama J, Poulain F, Vanderbilt J, Hawgood S. Am J Respir Crit Care Med. 1998;157:A561. (abstr.).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

