Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression
- PMID: 9756743
- PMCID: PMC105859
- DOI: 10.1128/AAC.42.10.2467
Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression
Abstract
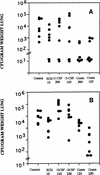
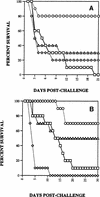
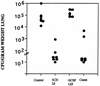
Outbred ICR mice were immune suppressed either with hydrocortisone or with 5-fluorouracil and were infected intranasally with Aspergillus fumigatus. Beginning 3 days before infection some groups of mice were given recombinant human granulocyte colony-stimulating factor (G-CSF), SCH56592 (an antifungal triazole), or both. Corticosteroid-pretreated mice responded to SCH56592 and had reduced counts in lung tissue and prolonged survival. In these mice, G-CSF strongly antagonized the antifungal activity of SCH56592. Animals treated with both agents developed large lung abscesses with polymorphonuclear leukocytes and large amounts of Aspergillus. In contrast, mice made neutropenic with 5-fluorouracil and then infected with A. fumigatus conidia benefited from either G-CSF or triazoles, and the effect of the combination was additive rather than antagonistic. Host predisposing factors contribute in different ways to the outcome of growth factor therapy in aspergillosis.
Figures



References
-
- Adelman B A, Bentman A, Rosenthal P, Smith B R, Bridges K R, Holmes W. Treatment of aspergillosis in leukemia. Ann Intern Med. 1979;91:323–324. - PubMed
-
- Albelda S M, Talbot G H, Gerson S L, Miller W T, Cassileth P A. Pulmonary cavitation and massive hemoptysis in invasive pulmonary aspergillosis: influence of bone marrow recovery in patients with acute leukemia. Am Rev Respir Dis. 1985;131:115–120. - PubMed
-
- Andriole, V. T. 1993. Infections with Aspergillus species. Clin. Infect. Dis. 17(Suppl. 2):S481–S486. - PubMed
-
- Berenguer J, Allende M C, Lee J W, Garret K, Lyman C, Ali N M, Bacher J, Pizzo P A, Walsh T J. Pathogenesis of pulmonary aspergillosis—granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am J Respir Crit Care Med. 1995;152:1079–1086. - PubMed
-
- Binder R E, Faling L J, Pugatch R D, Mahasaen C, Snider G L. Chronic necrotizing pulmonary aspergillosis: a discrete clinical entity. Medicine (Baltimore) 1982;61:109–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

