Efficacies of KY62 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous leishmaniasis and visceral leishmaniasis
- PMID: 9756753
- PMCID: PMC105886
- DOI: 10.1128/AAC.42.10.2542
Efficacies of KY62 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous leishmaniasis and visceral leishmaniasis
Abstract
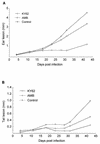
Current therapy for leishmaniasis is unsatisfactory because parenteral antimonial salts and pentamidine are associated with significant toxicity and failure rates. We examined the efficacy of KY62, a new, water-soluble, polyene antifungal, against cutaneous infection with Leishmania amazonensis and against visceral infection with Leishmania donovani in susceptible BALB/c mice. Mice were infected with L. amazonensis promastigotes in the ear pinna and in the tail and were treated with KY62 or amphotericin B. The cutaneous lesions showed a remarkable response to therapy with KY62 at a dose of 30 mg per kg of body weight per day. At this dose, the efficacy of KY62 was equivalent to or better than that of amphotericin B at 1 to 5 mg/kg/day. Mice infected intravenously with 10(7) L. donovani promastigotes and treated with KY62 showed a 4-log reduction in the parasite burden in the liver and spleen compared to untreated mice. These studies indicate potent activity of KY62 against experimental cutaneous leishmaniasis caused by L. amazoniensis and against experimental visceral leishmaniasis caused by L. donovani.
Figures




References
-
- Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. - PubMed
-
- Bjorvatn B, Neva F A. Experimental therapy of mice infected with Leishmania tropica. Am J Trop Med Hyg. 1979;28:480–485. - PubMed
-
- Castagnola E, Davidson R N, Fiore P, Tasso L, Rossi G, Mangraviti S, di Martino L, Scotti S, Cascio A, Pempinello R, Gradoni L, Giacchino R. Early efficacy of liposomal amphotericin B in the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1996;90:317–318. - PubMed
-
- Davidson R N, di Martino L, Gradoni L, Giacchino R, Gaeta G B, Pempinello R, Scotti S, Cascio A, Castagnola E, Maisto A, Gramiccia M, di Caprio D, Wilkinson R J, Bryceson A D. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22:938–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

