Development of a new cartridge radioimmunoassay for determination of intracellular levels of lamivudine triphosphate in the peripheral blood mononuclear cells of human immunodeficiency virus-infected patients
- PMID: 9756772
- PMCID: PMC105914
- DOI: 10.1128/AAC.42.10.2656
Development of a new cartridge radioimmunoassay for determination of intracellular levels of lamivudine triphosphate in the peripheral blood mononuclear cells of human immunodeficiency virus-infected patients
Abstract
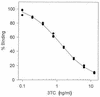
A new sensitive method for the measurement of lamivudine triphosphate (3TC-TP), the active intracellular metabolite of lamivudine in human cells in vivo, has been established. The procedure involves rapid separation of 3TC-TP by using Sep-Pak cartridges, dephosphorylation to 3TC by using acid phosphatase, and measurement by radioimmunoassay using a newly developed anti-3TC serum. The radioimmunoassay had errors of less than 21% and a cross-reactivity of less than 0.016% with a wide variety of other nucleoside analogs. The limit of quantitation of the assay for intracellular 3TC-TP was 0.195 ng/ml (0.212 pmol/10(6) cells), and a cell sample of only 4 million cells was ample for the assay. This procedure, combined with our previously developed method for measuring zidovudine (ZDV) metabolite levels, proved capable of measuring 3TC-TP, ZDV monophosphate (ZDV-MP) and ZDV triphosphate (ZDV-TP) in human immunodeficiency virus (HIV)-infected subjects treated with combination 3TC and ZDV therapy. In seven subjects, intracellular 3TC-TP levels ranged from 2.21 to 7.29 pmol/10(6) cells, while intracellular ZDV-MP and ZDV-TP levels ranged from <0. 01 to 1.76 and 0.01 to 0.07 pmol/10(6) cells, respectively. Concentrations of 3TC in plasma determined in these subjects ranged from 0.34 to 9.40 microM, which was about fivefold higher than ZDV levels in plasma of 0.04 to 1.4 microM. This is the first study to determine the intracellular levels of the active metabolites in HIV-infected subjects treated with this combination. These methods should prove very useful for in vivo pharmacodynamic studies of combination therapy.
Figures
References
-
- Dienstag J L, Perrillo R P, Schiff E R, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. - PubMed
-
- Fletcher C V, Kawle S P, Page L M, Remmel R P, Acosta E P, Henry K, Erice A, Balfour H H. Program and abstracts of the Fourth Conference on Retroviruses and Opportunistic Infections, Washington, D.C. 1997. Intracellular triphosphate concentrations of antiretroviral nucleosides as a determinant of clinical response in HIV-infected patients.
-
- Furman P A, Fyfe J A, Sinclair M H, Weinhold K, Rideout J L, Freemen F A, Lehrman S N, Bolognesi D P, Broder S, Mitsuya H, Barry D W. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. - PMC - PubMed
-
- Gao W-Y, Agbaria R, Driscoll J S, Mitsuya H. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem. 1994;269:12633–12638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical