Proteasome inhibitors block development of Plasmodium spp
- PMID: 9756786
- PMCID: PMC105928
- DOI: 10.1128/AAC.42.10.2731
Proteasome inhibitors block development of Plasmodium spp
Abstract
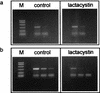
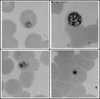
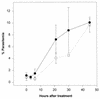
Proteasomes degrade most of the proteins inside eukaryotic cells, including transcription factors and regulators of cell cycle progression. Here we show that nanomolar concentrations of lactacystin, a specific irreversible inhibitor of the 20S proteasome, inhibit development of the exoerythrocytic and erythrocytic stages of the malaria parasite. Although lactacystin-treated Plasmodium berghei sporozoites are still invasive, their development into exoerythrocytic forms (EEF) is inhibited in vitro and in vivo. Erythrocytic schizogony of P. falciparum in vitro is also profoundly inhibited when drug treatment of the synchronized parasites is prior, but not subsequent, to the initiation of DNA synthesis, suggesting that the inhibitory effect of lactacystin is cell cycle specific. Lactacystin reduces P. berghei parasitemia in rats, but the therapeutic index is very low. Along with other studies showing that lactacystin inhibits stage-specific transformation in Trypanosoma and Entamoeba spp., these findings highlight the potential of proteasome inhibitors as drugs for the treatment of diseases caused by protozoan parasites.
Figures




References
-
- Aikawa M, Schwartz A, Uni S, Nussenzweig R, Hollingdale M. Ultrastructure of in vitro cultured exoerythrocytic stage of Plasmodium berghei in a hepatoma cell line. Am J Trop Med Hyg. 1984;33:792–799. - PubMed
-
- Briones M R S, Tsuji M, Nussenzweig V. The large differences in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter hepatocytes. Mol Biochem Parasitol. 1996;77:7–17. - PubMed
-
- Butler D, Maurice J, O’Brien C. Time to put malaria control on the global agenda. Nature. 1997;386:535–540. - PubMed
-
- Corey E J, Reichard G A. Total synthesis of lactacystin. J Am Chem Soc. 1992;114:10677–10678.
-
- Coux O, Tanaka K, Goldberg A L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

