Thermocrinis ruber gen. nov., sp. nov., A pink-filament-forming hyperthermophilic bacterium isolated from yellowstone national park
- PMID: 9758770
- PMCID: PMC106467
- DOI: 10.1128/AEM.64.10.3576-3583.1998
Thermocrinis ruber gen. nov., sp. nov., A pink-filament-forming hyperthermophilic bacterium isolated from yellowstone national park
Abstract
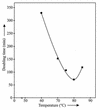
A novel hyperthermophilic bacterium was isolated from pink filamentous streamers (pink filaments) occurring in the upper outflow channel (temperature, 82 to 88 degreesC) of Octopus Spring in Yellowstone National Park, Wyo. The gram-negative cells grew at low salinity at temperatures up to 89 degreesC in the neutral to alkaline pH range. Depending on the culture conditions, the organisms occurred as single motile rods, as aggregates, or as long filaments that formed streamer-like cell masses. The novel isolate grew chemolithoautotrophically with hydrogen, thiosulfate, and elemental sulfur as electron donors and oxygen as the electron acceptor. Alternatively, under aerobic conditions, formate and formamide served as sole energy and carbon sources. The novel isolate had a 16S rRNA sequence closely related to the 16S rRNA sequence obtained from uncultivated pink filaments. It represents a new genus in the order Aquificales, the type species of which we name Thermocrinis ruber (type strain, OC 1/4 [= DSM 12173]).
Figures






References
-
- Alfredsson G A, Ingason A, Kristjansson J K. Growth of thermophilic obligately autotrophic hydrogen-oxidizing bacteria on thiosulfate. Lett Appl Microbiol. 1986;2:21–23.
-
- Aragno M. Thermophilic, aerobic, hydrogen-oxidizing (Knallgas) bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. Vol. 4. New York, N.Y: Springer-Verlag; 1992. pp. 3917–3933.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

