Application of molecular biological techniques to a seasonal study of ammonia oxidation in a eutrophic freshwater lake
- PMID: 9758784
- PMCID: PMC106508
- DOI: 10.1128/AEM.64.10.3674-3682.1998
Application of molecular biological techniques to a seasonal study of ammonia oxidation in a eutrophic freshwater lake
Abstract
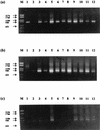
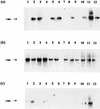
The autotrophic ammonia-oxidizing bacteria in a eutrophic freshwater lake were studied over a 12-month period. Numbers of ammonia oxidisers in the lakewater were small throughout the year, and tangential-flow concentration was required to obtain meaningful estimates of most probable numbers. Sediments from littoral and profundal sites supported comparatively large populations of these bacteria, and the nitrification potential was high, particularly in summer samples from the littoral sediment surface. In enrichment cultures, lakewater samples nitrified at low (0.67 mM) ammonium concentrations only whereas sediment samples exhibited nitrification at high (12.5 mM) ammonium concentrations also. Enrichments at low ammonium concentration did not nitrify when inoculated into high-ammonium medium, but the converse was not true. This suggests that the water column contains a population of ammonia oxidizers that is sensitive to high ammonium concentrations. The observation of nitrification at high ammonium concentration by isolates from some winter lakewater samples, identified as nitrosospiras by 16S rRNA probing, is consistent with the hypothesis that sediment ammonia oxidizers enter the water column at overturn. With only one exception, nested PCR amplification enabled the detection of Nitrosospira 16S rDNA in all samples, but Nitrosomonas (N. europaea-eutropha lineage) 16S rDNA was never obtained. However, the latter were part of the sediment and water column communities, because their 16S rRNA could be detected by specific oligonucleotide probing of enrichment cultures. Furthermore, a specific PCR amplification regime for the Nitrosomonas europaea ammonia monooxygenase gene (amoA) yielded positive results when applied directly to sediment and lakewater samples. Patterns of Nitrosospira and Nitrosomonas detection by 16S rRNA oligonucleotide probing of sediment enrichment cultures were complex, but lakewater enrichments at low ammonium concentration were positive for nitrosomonads and not nitrosospiras. Analysis of enrichment cultures has therefore provided evidence for the existence of subpopulations within the lake ammonia-oxidizing community distinguishable on the basis of ammonium tolerance and possibly showing a seasonal distribution between the sediment and water column.
Figures

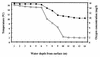
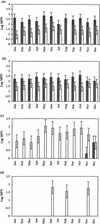
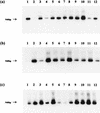
 ) and 0.67 mM (□) NH4(SO4)2 medium from littoral sediment (a), profundal sediment (b), a concentrated lakewater sample (c) and an untreated lakewater sample (d). Thin bars indicate 95% confidence limits.
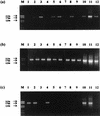
) and 0.67 mM (□) NH4(SO4)2 medium from littoral sediment (a), profundal sediment (b), a concentrated lakewater sample (c) and an untreated lakewater sample (d). Thin bars indicate 95% confidence limits.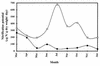
References
-
- Bendschneider K, Robinson R J. A new spectrophotometric determination of nitrite in seawater. J Mar Res. 1952;2:87–96.
-
- Hall G H. Nitrification in lakes. In: Prosser J I, editor. Nitrification. Oxford, England: IRL Press; 1986. p. 99.
-
- Hall G H, Jeffries C. The contribution of nitrification in the water column and profundal sediments to the total oxygen deficit of the hypolimnion of a mesotrophic lake (Grasmere, English Lake District) Microb Ecol. 1984;10:37–46. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

