Determination of the biomasses of small bacteria at low concentrations in a mixture of species with forward light scatter measurements by flow cytometry
- PMID: 9758817
- PMCID: PMC106576
- DOI: 10.1128/AEM.64.10.3900-3909.1998
Determination of the biomasses of small bacteria at low concentrations in a mixture of species with forward light scatter measurements by flow cytometry
Abstract
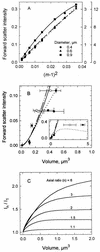
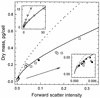
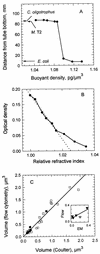
The forward light scatter intensity of bacteria analyzed by flow cytometry varied with their dry mass, in accordance with theory. A standard curve was formulated with Rayleigh-Gans theory to accommodate cell shape and alignment. It was calibrated with an extinction-culture isolate of the small marine organism Cycloclasticus oligotrophus, for which dry weight was determined by CHN analysis and 14C-acetate incorporation. Increased light scatter intensity due to formaldehyde accumulation in preserved cells was included in the standard curve. When differences in the refractive indices of culture media and interspecies differences in the effects of preservation were taken into account, there was agreement between cell mass obtained by flow cytometry for various bacterial species and cell mass computed from Coulter Counter volume and buoyant density. This agreement validated the standard curve and supported the assumption that cells were aligned in the flow stream. Several subpopulations were resolved in a mixture of three species analyzed according to forward light scatter and DNA-bound DAPI (4', 6-diamidino-2-phenylindole) fluorescence intensity. The total biomass of the mixture was 340 &mgr;g/liter. The lowest value for mean dry mass, 0.027 +/- 0.008 pg/cell, was for the subpopulation of C. oligotrophus containing cells with a single chromosome. Calculations from measurements of dry mass, Coulter Counter volume, and buoyant density revealed that the dry weight of the isolate was 14 to 18% of its wet weight, compared to 30% for Escherichia coli. The method is suitable for cells with 0.005 to about 1.2 pg of dry weight at concentrations of as low as 10(3) cells/ml and offers a unique capability for determining biomass distributions in mixed bacterial populations.
Figures

References
-
- Ackleson S G, Spinrad R W. Size and refractive index of individual marine particulates: a flow cytometric approach. Appl Optics. 1988;27:1270–1277. - PubMed
-
- Allman R, Hann A C, Phillips A P, Martin K L, Lloyd D. Growth of Azotobacter vinelandii with correlation of Coulter cell size, flow cytometric parameters and ultrastructure. Cytometry. 1990;11:822–831. - PubMed
-
- Baldwin W W, Myer R, Powell N, Anderson E, Koch A L. Buoyant density of Escherichia coli is determined solely by the osmolarity of the culture medium. Arch Microbiol. 1995;164:155–157. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

