Purification and characterization of an NAD-malic enzyme from Bradyrhizobium japonicum A1017
- PMID: 9758846
- PMCID: PMC106605
- DOI: 10.1128/AEM.64.10.4073-4075.1998
Purification and characterization of an NAD-malic enzyme from Bradyrhizobium japonicum A1017
Abstract
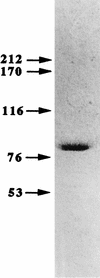
An NAD-malic enzyme was purified to homogeneity from Bradyrhizobium japonicum A1017, and its molecular characteristics were surveyed. The enzyme exhibited native and subunit molecular masses of 388 and 85 kDa, respectively, suggesting that it exists as a homotetramer, and was activated by metabolic intermediates in glycolysis. The role of the enzyme in bacteroids' carbon metabolism is discussed.
Figures
References
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chen F, Okabe Y, Osano K, Tajima S. Purification and characterization of the NADP-malic enzyme from Bradyrhizobium japonicum A1017. Biosci Biotech Biochem. 1997;61:384–386. - PubMed
-
- Copeland L, Quinnell R G, Day D A. Malic enzyme activity in bacteroids from soybean nodules. J Gen Microbiol. 1989;135:2005–2011.
-
- Day D A, Quinnell R G, Bergersen F J. An hypothesis on the role of malic enzyme in symbiotic nitrogen fixation in soybean nodules. In: Graham P H, Sadowsky M J, Vance C P, editors. Symbiotic nitrogen fixation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 159–164.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources