Permeation and gating of an inwardly rectifying potassium channel. Evidence for a variable energy well
- PMID: 9758862
- PMCID: PMC2229425
- DOI: 10.1085/jgp.112.4.433
Permeation and gating of an inwardly rectifying potassium channel. Evidence for a variable energy well
Abstract
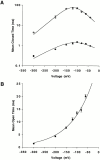
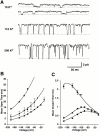
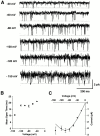
Permeation, gating, and their interrelationship in an inwardly rectifying potassium (K+) channel, ROMK2, were studied using heterologous expression in Xenopus oocytes. Patch-clamp recordings of single channels were obtained in the cell-attached mode. The gating kinetics of ROMK2 were well described by a model having one open and two closed states. One closed state was short lived (approximately 1 ms) and the other was longer lived (approximately 40 ms) and less frequent (approximately 1%). The long closed state was abolished by EDTA, suggesting that it was due to block by divalent cations. These closures exhibit a biphasic voltage dependence, implying that the divalent blockers can permeate the channel. The short closures had a similar biphasic voltage dependence, suggesting that they could be due to block by monovalent, permeating cations. The rate of entering the short closed state varied with the K+ concentration and was proportional to current amplitude, suggesting that permeating K+ ions may be related to the short closures. To explain the results, we propose a variable intrapore energy well model in which a shallow well may change into a deep one, resulting in a normally permeant K+ ion becoming a blocker of its own channel.
Figures
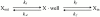









References
-
- Bennett WS, Jr, Steitz TA. Structure of a complex between yeast hexokinase A and glucose. II. Detailed comparisons of conformation and active site configuration with the native hexokinase B monomer and dimer. J Mol Biol. 1980;140:211–230. - PubMed
-
- Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+channel. II. Cloning and distribution of alternative forms. Am J Physiol. 1995;268:F1132–F1140. - PubMed

