Requirement of heavy neurofilament subunit in the development of axons with large calibers
- PMID: 9763431
- PMCID: PMC2132822
- DOI: 10.1083/jcb.143.1.195
Requirement of heavy neurofilament subunit in the development of axons with large calibers
Abstract
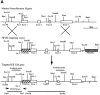
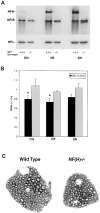
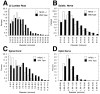
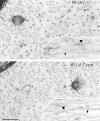
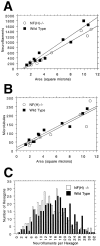
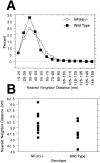
Neurofilaments (NFs) are prominent components of large myelinated axons. Previous studies have suggested that NF number as well as the phosphorylation state of the COOH-terminal tail of the heavy neurofilament (NF-H) subunit are major determinants of axonal caliber. We created NF-H knockout mice to assess the contribution of NF-H to the development of axon size as well as its effect on the amounts of low and mid-sized NF subunits (NF-L and NF-M respectively). Surprisingly, we found that NF-L levels were reduced only slightly whereas NF-M and tubulin proteins were unchanged in NF-H-null mice. However, the calibers of both large and small diameter myelinated axons were diminished in NF-H-null mice despite the fact that these mice showed only a slight decrease in NF density and that filaments in the mutant were most frequently spaced at the same interfilament distance found in control. Significantly, large diameter axons failed to develop in both the central and peripheral nervous systems. These results demonstrate directly that unlike losing the NF-L or NF-M subunits, loss of NF-H has only a slight effect on NF number in axons. Yet NF-H plays a major role in the development of large diameter axons.
Figures



Comment in
-
Gene targeting studies begin to reveal the function of neurofilament proteins.J Cell Biol. 1998 Oct 5;143(1):1-4. doi: 10.1083/jcb.143.1.1. J Cell Biol. 1998. PMID: 9763415 Free PMC article. Review. No abstract available.
References
-
- Balin BJ, Clark EA, Trojanowski JQ, Lee VM-Y. Neurofilament reassembly in vitro: biochemical, morphological and immuno-electron microscopic studies employing antibodies to defined epitopes. Brain Res. 1991;556:181–195. - PubMed
-
- Balin BJ, Lee VM-Y. Individual neurofilament subunits reassembled in vitro exhibit unique biochemical, morphological and immunological properties. Brain Res. 1991;556:196–208. - PubMed
-
- Berthold, C.-H. 1978. Morphology of normal peripheral axons. In Physiology and Pathobiology of Axons. S.G. Waxman, editor. Raven Press, New York. 3–63.
-
- Carter J, Gragerov A, Konvicka K, Elder G, Weinstein H, Lazzarini RA. Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J Biol Chem. 1998;273:5101–5108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

