A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae
- PMID: 9763445
- PMCID: PMC25555
- DOI: 10.1091/mbc.9.10.2803
A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae
Abstract
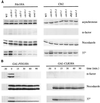
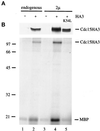
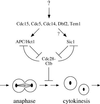
Exit from mitosis requires the inactivation of mitotic cyclin-dependent kinase-cyclin complexes, primarily by ubiquitin-dependent cyclin proteolysis. Cyclin destruction is regulated by a ubiquitin ligase known as the anaphase-promoting complex (APC). In the budding yeast Saccharomyces cerevisiae, members of a large class of late mitotic mutants, including cdc15, cdc5, cdc14, dbf2, and tem1, arrest in anaphase with a phenotype similar to that of cells expressing nondegradable forms of mitotic cyclins. We addressed the possibility that the products of these genes are components of a regulatory network that governs cyclin proteolysis. We identified a complex array of genetic interactions among these mutants and found that the growth defect in most of the mutants is suppressed by overexpression of SPO12, YAK1, and SIC1 and is exacerbated by overproduction of the mitotic cyclin Clb2. When arrested in late mitosis, the mutants exhibit a defect in cyclin-specific APC activity that is accompanied by high Clb2 levels and low levels of the anaphase inhibitor Pds1. Mutant cells arrested in G1 contain normal APC activity. We conclude that Cdc15, Cdc5, Cdc14, Dbf2, and Tem1 cooperate in the activation of the APC in late mitosis but are not required for maintenance of that activity in G1.
Figures




References
-
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
-
- Amon A, Tyers M, Futcher B, Nasmyth K. Mechanisms that help the yeast cell cycle clock tick—G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell. 1993;74:993–1007. - PubMed
-
- Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

