Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells
- PMID: 9763451
- PMCID: PMC25565
- DOI: 10.1091/mbc.9.10.2905
Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells
Abstract
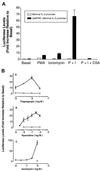
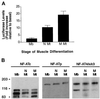
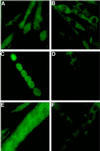
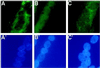
The widely used immunosuppressant cyclosporine A (CSA) blocks nuclear translocation of the transcription factor, NF-AT (nuclear factor of activated T cells), preventing its activity. mRNA for several NF-AT isoforms has been shown to exist in cells outside of the immune system, suggesting a possible mechanism for side effects associated with CSA treatment. In this study, we demonstrate that CSA inhibits biochemical and morphological differentiation of skeletal muscle cells while having a minimal effect on proliferation. Furthermore, in vivo treatment with CSA inhibits muscle regeneration after induced trauma in mice. These results suggest a role for NF-AT-mediated transcription outside of the immune system. In subsequent experiments, we examined the activation and cellular localization of NF-AT in skeletal muscle cells in vitro. Known pharmacological inducers of NF-AT in lymphoid cells also stimulate transcription from an NF-AT-responsive reporter gene in muscle cells. Three isoforms of NF-AT (NF-ATp, c, and 4/x/c3) are present in the cytoplasm of muscle cells at all stages of myogenesis tested. However, each isoform undergoes calcium-induced nuclear translocation from the cytoplasm at specific stages of muscle differentiation, suggesting specificity among NF-AT isoforms in gene regulation. Strikingly, one isoform (NF-ATc) can preferentially translocate to a subset of nuclei within a single multinucleated myotube. These results demonstrate that skeletal muscle cells express functionally active NF-AT proteins and that the nuclear translocation of individual NF-AT isoforms, which is essential for the ability to coordinate gene expression, is influenced markedly by the differentiation state of the muscle cell.
Figures



References
-
- Berman SA, Bursztajn S, Bowen B, Gilbert W. Localization of an acetylcholine receptor intron to the nuclear membrane. Science. 1990;247:212–214. - PubMed
-
- Boss V, Abbott KA, Wang X-F, Pavlath GK, Murphy TJ. Expression of the cyclosporin A-sensitive factor NFAT in cultured vascular smooth muscle cells: differential induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors. J Biol Chem. 1998;273:19664–19671. - PubMed
-
- Boss V, Talpade DJ, Murphy TJ. Induction of NFAT-mediated transcription by Gq-coupled receptors in lymphoid and non-lymphoid cells. J Biol Chem. 1996;271:10429–10432. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Changeux JP. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991;3:413–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

