Activity-dependent modulation of glutamate receptors by polyamines
- PMID: 9763464
- PMCID: PMC6792845
- DOI: 10.1523/JNEUROSCI.18-20-08175.1998
Activity-dependent modulation of glutamate receptors by polyamines
Abstract
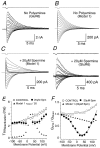
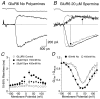
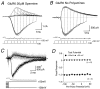
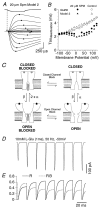
The mechanisms by which polyamines block AMPA and kainate receptors are not well understood, but it has been generally assumed that they act as open-channel blockers. Consistent with this, voltage-jump relaxation analysis of GluR6 equilibrium responses to domoate could be well fit, assuming that spermine, spermidine, and philanthotoxin are weakly permeable open-channel blockers. Analysis of rate constants for binding and dissociation of polyamines indicated that the voltage dependence of block arose primarily from changes in koff rather than kon. Experiments with changes in Na concentration further indicate that the voltage dependence of polyamine block was governed by ion flux via open channels. However, responses to 1 msec applications of L-Glu revealed slow voltage-dependent rise-times, suggesting that polyamines additionally bind to closed states. A kinetic model, which included closed-channel block, reproduced these observations but required that polyamines accelerate channel closure either through an allosteric mechanism or by emptying the pore of permeant ions. Simulations with this model reveal that polyamine block confers novel activity-dependent regulation on calcium-permeable AMPA and kainate receptor responses.
Figures


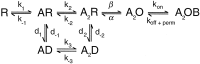

References
-
- Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+-channel. Science. 1996;271:653–656. - PubMed
-
- Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77:309–323. - PubMed
-
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
