Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex
- PMID: 9763491
- PMCID: PMC6792829
- DOI: 10.1523/JNEUROSCI.18-20-08485.1998
Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex
Abstract
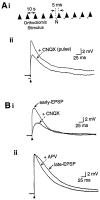
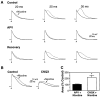
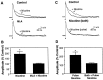
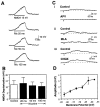
The neurotransmitters acetylcholine (ACh) and glutamate have been separately implicated in synaptic plasticity during development of sensory neocortex. Here we show that these neurotransmitters can, in fact, act synergistically via their actions at nicotinic ACh receptors (nAChRs) and NMDA receptors, respectively. To determine how activation of nAChRs modifies glutamatergic EPSPs, we made whole-cell recordings from visualized pyramidal neurons in slices of rat auditory cortex. Pulsed (pressure) ejection of nicotine onto apical dendrites selectively enhanced EPSPs mediated by NMDA receptors without affecting AMPA/kainate (AMPA/KA) receptor-mediated EPSPs. The enhancement occurred during a transient, postnatal period of heightened cholinergic function [neurons tested on postnatal day 8-16 (P8-16)], and not in the mature cortex (>P19). Three related findings indicated the mechanism of action: (1) The specific alpha7 nAChR antagonist methyllycaconitine citrate (MLA) blocked the effect of nicotine; (2) pulsed nicotine did not enhance postsynaptic depolarizations induced by iontophoretically applied NMDA; and (3) bath exposure to nicotine for several minutes produced apparent nAChR desensitization and precluded enhancement of EPSPs by pulsed nicotine. Together, the data suggest that nicotine acts at rapidly desensitizing, presynaptic alpha7 nAChRs to increase glutamate release onto postsynaptic NMDA receptors. The synergistic actions mediated by alpha7 nAChRs and NMDA receptors may contribute to experience-dependent synaptic plasticity in sensory neocortex during early postnatal life.
Figures


References
-
- Agmon A, O’Dowd DK. NMDA receptor-mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J Neurophysiol. 1992;68:345–349. - PubMed
-
- Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat brain. V. α-Bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996;278:1460–1471. - PubMed
-
- Aramakis VB, Bandrowski AE, Ashe JH. Activation of muscarinic receptors modulates NMDA-receptor mediated responses in auditory cortex. Exp Brain Res. 1997;113:484–496. - PubMed
-
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. - PubMed
-
- Bekkers J, Stevens C. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
