Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness
- PMID: 9763610
- PMCID: PMC2212487
- DOI: 10.1084/jem.188.7.1307
Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness
Abstract
Interleukin (IL)-9, a pleiotropic cytokine produced by the Th2 subset of T lymphocytes has been proposed as product of a candidate gene responsible for asthma. Its wide range of biological functions on many cell types involved in the allergic immune response suggests a potentially important role in the complex pathogenesis of asthma. To investigate the contributions of IL-9 to airway inflammation and airway hyperresponsiveness in vivo, we created transgenic mice in which expression of the murine IL-9 cDNA was regulated by the rat Clara cell 10 protein promoter. Lung selective expression of IL-9 caused massive airway inflammation with eosinophils and lymphocytes as predominant infiltrating cell types. A striking finding was the presence of increased numbers of mast cells within the airway epithelium of IL-9-expressing mice. Other impressive pathologic changes in the airways were epithelial cell hypertrophy associated with accumulation of mucus-like material within nonciliated cells and increased subepithelial deposition of collagen. Physiologic evaluation of IL-9-expressing mice demonstrated normal baseline airway resistance and markedly increased airway hyperresponsiveness to inhaled methacholine. These findings strongly support an important role for IL-9 in the pathogenesis of asthma.
Figures
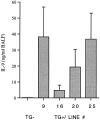
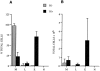
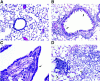






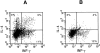

References
-
- Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. - PubMed
-
- Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995;13:1–6. - PubMed
-
- Busse WW, Coffman RL, Gelfand EW, Kay AB, Rosenwasser LJ. Mechanisms of persistant airway inflammation. Am J Respir Crit Care Med. 1995;152:388–393. - PubMed
-
- Barnes PJ. Cytokines as mediators of chronic asthma. Am J Respir Crit Care Med. 1994;150:S42–S49. - PubMed
-
- Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Activation and regulation of chemokines in allergic airway inflammation. J Leukocyte Biol. 1996;59:13–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

