The interleukin 2 receptor alpha chain/CD25 promoter is a target for nuclear factor of activated T cells
- PMID: 9763616
- PMCID: PMC2212486
- DOI: 10.1084/jem.188.7.1369
The interleukin 2 receptor alpha chain/CD25 promoter is a target for nuclear factor of activated T cells
Abstract
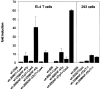
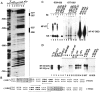
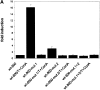
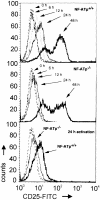
The expression of the murine interleukin (IL)-2 receptor alpha chain/CD25 is strongly induced at the transcriptional level after T cell activation. We show here that nuclear factor of activated T cell (NF-AT) factors are involved in the control of CD25 promoter induction in T cells. NF-ATp and NF-ATc bind to two sites around positions -585 and -650 located upstream of the proximal CD25 promoter. Immediately 3' from these NF-AT motifs, nonconsensus sites are located for the binding of AP-1-like factors. Mutations of sites that suppress NF-AT binding impair the induction and strong NF-ATp-mediated transactivation of the CD25 promoter in T cells. In T lymphocytes from NF-ATp-deficient mice, the expression of CD25 is severely impaired, leading to a delayed IL-2 receptor expression after T cell receptor (TCR)/CD3 stimulation. Our data indicate an important role for NF-AT in the faithful expression of high affinity IL-2 receptors and a close link between the TCR-mediated induction of IL-2 and IL-2 receptor alpha chain promoters, both of which are regulated by NF-AT factors.
Figures

References
-
- Minami J, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. - PubMed
-
- Sperisen P, Wang SM, Soldaini E, Pla M, Rusterholz C, Bucher P, Corthesy P, Reichenbach P, Nabholz M. Mouse interleukin-2 receptor α gene expression. Interleukin-1 and interleukin-2 control transcription via distinct cis-acting elements. J Biol Chem. 1995;277:10743–10752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

