An ether -à-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts
- PMID: 9763622
- PMCID: PMC2231207
- DOI: 10.1111/j.1469-7793.1998.317be.x
An ether -à-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts
Abstract
1. Two early signs of human myoblast commitment to fusion are membrane potential hyperpolarization and concomitant expression of a non-inactivating delayed rectifier K+ current, IK(NI). This current closely resembles the outward K+ current elicited by rat ether-à-go-go (r-eag) channels in its range of potential for activation and unitary conductance. 2. It is shown that activation kinetics of IK(NI), like those of r-eag, depend on holding potential and on [Mg2+]o, and that IK(NI), like r-eag, is reversibly inhibited by a rise in [Ca2+]i. 3. Forced expression of an isolated human ether-à-go-go K+ channel (h-eag) cDNA in undifferentiated myoblasts generates single-channel and whole-cell currents with remarkable similarity to IK(NI). 4. h-eag current (Ih-eag) is reversibly inhibited by a rise in [Ca2+]i, and the activation kinetics depend on holding potential and [Mg2+]o. 5. Forced expression of h-eag hyperpolarizes undifferentiated myoblasts from -9 to -50 mV, the threshold for the activation of both Ih-eag and IK(NI). Similarly, the higher the density of IK(NI), the more hyperpolarized the resting potential of fusion-competent myoblasts. 6. It is concluded that h-eag constitutes the channel underlying IK(NI) and that it contributes to the hyperpolarization of fusion-competent myoblasts. To our knowledge, this is the first demonstration of a physiological role for a mammalian eag K+ channel.
Figures
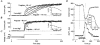
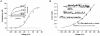


References
-
- Baroffio A, Aubry JP, Kaelin A, Krause RM, Hamann M, Bader CR. Purification of human muscle satellite cells by flow cytometry. Muscle and Nerve. 1993;16:498–505. - PubMed
-
- Binggeli R, Weinstein RC. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. Journal of Theoretical Biology. 1986;123:377–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

