Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: association of IL-10 production with CD28-mediated immune responsiveness
- PMID: 9764607
- PMCID: PMC1905077
- DOI: 10.1046/j.1365-2249.1998.00689.x
Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: association of IL-10 production with CD28-mediated immune responsiveness
Abstract
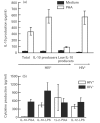
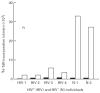
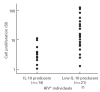
Immune unresponsiveness in HIV-1 infection can result from impaired signals delivered by the costimulatory CD28-B7 pathway and the altered production of immunoregulatory cytokines, in particular IL-10, whose production is altered in HIV-1 infection. In this study we investigate IL-10 regulation in T cells and monocytes from HIV+ individuals, and its association with CD28-mediated T cell proliferation. IL-10 production as analysed in T cell- and monocyte-depleted peripheral blood mononuclear cells (PBMC), and by intracellular staining at the single-cell level, reveals a defect in IL-10 production by CD4+ and CD8+ T cells, whereas monocytes constitute the major IL-10-producing cell type. To investigate the impact of IL-10 on immune responsiveness, CD28-mediated proliferative responses in HIV+ individuals were correlated with PHA-induced IL-10 production. CD4+ T cells expressed CD28, yet exhibited markedly reduced CD28-mediated cell proliferation. This CD28-mediated CD4+ T cell proliferation was found to be inversely associated with the levels of PHA-induced IL-10 production and could be restored, at least in part, by anti-IL-10 antibodies. These results suggest that IL-10 production is differentially regulated in T cells and monocytes of HIV+ individuals, and that IL-10 may have a role in inducing immune unresponsiveness by modulating the CD28-B7 pathway.
Figures

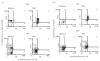

References
-
- Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–34. - PubMed
-
- Giorgi JV, Fahey JL, Smith DC, Hultin LE, Cheng HL, Mitsuyasu RT, Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987;138:3725–30. - PubMed
-
- Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

