Caffeine based measures of CYP1A2 activity correlate with oral clearance of tacrine in patients with Alzheimer's disease
- PMID: 9764962
- PMCID: PMC1873677
- DOI: 10.1046/j.1365-2125.1998.00776.x
Caffeine based measures of CYP1A2 activity correlate with oral clearance of tacrine in patients with Alzheimer's disease
Abstract
Aims: To study the potential utility of caffeine based probes of CYP1A2 enzyme activity in predicting the pharmokinetics of tacrine in patients with Alzheimer's disease.
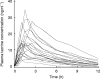
Methods: The pharmokinetics of a single 40 mg oral dose of tacrine were measured in 19 patients with Alzheimer's disease. Each patient also received 2 mg kg(-1) [13C-3-methyl] caffeine orally and had breath and urine samples collected.
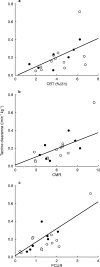
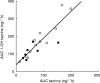
Results: Tacrine oral clearance (CL F(-1) kg(-1)), which varied 15-fold among the patients, correlated significantly with the 2 h total production of 13CO2 in breath (r=0.56, P=0.01), and with each of two commonly used urinary caffeine metabolite ratios: the 'paraxanthine/caffeine ratio' (1,7X + 1, 7U)/1,3,7X) (r=0.76, P=0.0002) and the 'caffeine metabolic ratio' (AFMU + 1X + 1U)/1, 7U)(r=0.76, P=0.0001).
Conclusions: These observations support a central role for CYP1A2 in the in vivo disposition of tacrine and the potential for drug interactions when tacrine treated patients receive known inducers or inhibitors of this enzyme. The magnitude of the correlations we observed, however, are probably not sufficient to be clinically useful in individualizing tacrine therapy.
Figures
References
-
- Farlow M. Management of Alzheimer's Disease: Today's Options and Tomorrow's Opportunities. Alz Dis Assoc Disord. 1994;8(Suppl 2):S50–S57.
-
- Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. JAMA. 1994;271:985–991. - PubMed
-
- Parke-Davis Pharmaceuticals Warner Lambert. Cognex (tacrine) package insert. Parke Davis Pharm. 1994.
-
- Ford JM, Truman CA, Wilcock GK, Roberts CJ. Serum concentrations of tacrine hydrochloride predict its adverse effects in Alzheimer's disease. Clin Pharmacol Ther. 1993;53:691–695. - PubMed
-
- Hartvig P, Askmark H, Aquilonius SM, Wiklund L, Lindstrom B. Clinical Pharmacokinetics of intravenous and oral 9-amino-1,2,3,4-tetrahydroacridine, Tacrine. Eur J Clin Pharmacol. 1990;38:259–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

