The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro
- PMID: 9765412
- PMCID: PMC110284
- DOI: 10.1128/JVI.72.11.8705-8709.1998
The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro
Abstract
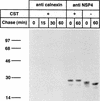
Calnexin is an endoplasmic reticulum (ER)-associated molecular chaperone proposed to promote folding and assembly of glycoproteins that traverse the secretory pathway in eukaryotic cells. In this study we examined if calnexin interacts with the ER-associated luminal (VP7) and transmembrane (NSP4) proteins of rotavirus. Only glycosylated NSP4 interacted with calnexin and did so in a time-dependent manner (half-life, 20 min). In vitro translation experiments programmed with gene 10 of rhesus rotavirus confirmed that calnexin recognizes only glycosylated NSP4. Castanospermine (a glucosidase I and II inhibitor) experiments established that calnexin associates only with partly deglucosylated (di- or monoglucosylated) NSP4. Furthermore, enzymatic removal of the remaining glucose residues on the N-linked glycan units was essential to disengage the NSP4-calnexin complex. Novel experiments with castanospermine revealed that glucose trimming and the calnexin-NSP4 interaction were not critical for the assembly of infectious virus.
Figures




Similar articles
-
COPII Vesicle Transport Is Required for Rotavirus NSP4 Interaction with the Autophagy Protein LC3 II and Trafficking to Viroplasms.J Virol. 2019 Dec 12;94(1):e01341-19. doi: 10.1128/JVI.01341-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597778 Free PMC article.
-
Carbohydrates facilitate correct disulfide bond formation and folding of rotavirus VP7.J Virol. 1998 May;72(5):3887-92. doi: 10.1128/JVI.72.5.3887-3892.1998. J Virol. 1998. PMID: 9557673 Free PMC article.
-
Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum.Cell. 1995 May 5;81(3):425-33. doi: 10.1016/0092-8674(95)90395-x. Cell. 1995. PMID: 7736594
-
Rotavirus protein expression is important for virus assembly and pathogenesis.Arch Virol Suppl. 1996;12:69-77. doi: 10.1007/978-3-7091-6553-9_8. Arch Virol Suppl. 1996. PMID: 9015103 Review.
-
How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea?Virol J. 2007 Mar 21;4:31. doi: 10.1186/1743-422X-4-31. Virol J. 2007. PMID: 17376232 Free PMC article. Review.
Cited by
-
Conformational Differences Unfold a Wide Range of Enterotoxigenic Abilities Exhibited by rNSP4 Peptides from Different Rotavirus Strains.Open Virol J. 2011;5:124-35. doi: 10.2174/1874357901105010124. Epub 2011 Nov 10. Open Virol J. 2011. PMID: 22253650 Free PMC article.
-
The roles of direct recognition by animal lectins in antiviral immunity and viral pathogenesis.Molecules. 2015 Jan 29;20(2):2272-95. doi: 10.3390/molecules20022272. Molecules. 2015. PMID: 25642837 Free PMC article. Review.
-
Rotavirus non-structural proteins: structure and function.Curr Opin Virol. 2012 Aug;2(4):380-8. doi: 10.1016/j.coviro.2012.06.003. Epub 2012 Jul 11. Curr Opin Virol. 2012. PMID: 22789743 Free PMC article. Review.
-
N-Glycosylation of Rotavirus NSP4 Protein Affects Viral Replication and Pathogenesis.J Virol. 2023 Jan 31;97(1):e0186122. doi: 10.1128/jvi.01861-22. Epub 2023 Jan 4. J Virol. 2023. PMID: 36598201 Free PMC article.
-
Endoplasmic reticulum chaperones are involved in the morphogenesis of rotavirus infectious particles.J Virol. 2008 Jun;82(11):5368-80. doi: 10.1128/JVI.02751-07. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385250 Free PMC article.
References
-
- Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

