Heterogeneous nuclear ribonucleoprotein L interacts with the 3' border of the internal ribosomal entry site of hepatitis C virus
- PMID: 9765422
- PMCID: PMC110294
- DOI: 10.1128/JVI.72.11.8782-8788.1998
Heterogeneous nuclear ribonucleoprotein L interacts with the 3' border of the internal ribosomal entry site of hepatitis C virus
Abstract
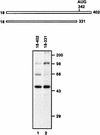
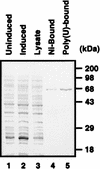
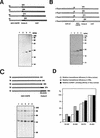
Translation initiation of hepatitis C virus (HCV) RNA occurs by internal entry of a ribosome into the 5' nontranslated region in a cap-independent manner. The HCV RNA sequence from about nucleotide 40 up to the N terminus of the coding sequence of the core protein is required for efficient internal initiation of translation, though the precise border of the HCV internal ribosomal entry site (IRES) has yet to be determined. Several cellular proteins have been proposed to direct HCV IRES-dependent translation by binding to the HCV IRES. Here we report on a novel cellular protein that specifically interacts with the 3' border of the HCV IRES in the core-coding sequence. This protein with an apparent molecular mass of 68 kDa turned out to be heterogeneous nuclear ribonucleoprotein L (hnRNP L). The binding of hnRNP L to the HCV IRES correlates with the translational efficiencies of corresponding mRNAs. This finding suggests that hnRNP L may play an important role in the translation of HCV mRNA through the IRES element.
Figures



Similar articles
-
hnRNP L is required for the translation mediated by HCV IRES.Biochem Biophys Res Commun. 2009 Jan 16;378(3):584-8. doi: 10.1016/j.bbrc.2008.11.091. Epub 2008 Dec 4. Biochem Biophys Res Commun. 2009. PMID: 19061868
-
RNA-binding protein hnRNP D modulates internal ribosome entry site-dependent translation of hepatitis C virus RNA.J Virol. 2008 Dec;82(24):12082-93. doi: 10.1128/JVI.01405-08. Epub 2008 Oct 8. J Virol. 2008. PMID: 18842733 Free PMC article.
-
A peptide derived from RNA recognition motif 2 of human la protein binds to hepatitis C virus internal ribosome entry site, prevents ribosomal assembly, and inhibits internal initiation of translation.J Virol. 2005 Aug;79(15):9842-53. doi: 10.1128/JVI.79.15.9842-9853.2005. J Virol. 2005. PMID: 16014945 Free PMC article.
-
Translation regulation by ribosomes: Increased complexity and expanded scope.RNA Biol. 2016 Sep;13(9):748-55. doi: 10.1080/15476286.2015.1107701. Epub 2015 Oct 29. RNA Biol. 2016. PMID: 26513496 Free PMC article. Review.
-
Hepatitis C virus RNA translation.Curr Top Microbiol Immunol. 2013;369:143-66. doi: 10.1007/978-3-642-27340-7_6. Curr Top Microbiol Immunol. 2013. PMID: 23463200 Review.
Cited by
-
Quantitative analysis of the hepatitis C virus replication complex.J Virol. 2005 Nov;79(21):13594-605. doi: 10.1128/JVI.79.21.13594-13605.2005. J Virol. 2005. PMID: 16227280 Free PMC article.
-
Viral and cellular proteins involved in coronavirus replication.Curr Top Microbiol Immunol. 2005;287:95-131. doi: 10.1007/3-540-26765-4_4. Curr Top Microbiol Immunol. 2005. PMID: 15609510 Free PMC article. Review.
-
cis-Acting RNA elements in the hepatitis C virus RNA genome.Virus Res. 2015 Aug 3;206:90-8. doi: 10.1016/j.virusres.2014.12.029. Epub 2015 Jan 7. Virus Res. 2015. PMID: 25576644 Free PMC article. Review.
-
Mechanism of ribosome recruitment by hepatitis C IRES RNA.RNA. 2001 Feb;7(2):194-206. doi: 10.1017/s1355838201001790. RNA. 2001. PMID: 11233977 Free PMC article.
-
Binding of hnRNP L to the pre-mRNA processing enhancer of the herpes simplex virus thymidine kinase gene enhances both polyadenylation and nucleocytoplasmic export of intronless mRNAs.Mol Cell Biol. 2005 Aug;25(15):6303-13. doi: 10.1128/MCB.25.15.6303-6313.2005. Mol Cell Biol. 2005. PMID: 16024770 Free PMC article.
References
-
- Dreyfuss G, Matunis M J, Pinol-Roma S, Burd C G. HnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

