Vaccinia virus protein synthesis has a low requirement for the intact translation initiation factor eIF4F, the cap-binding complex, within infected cells
- PMID: 9765426
- PMCID: PMC110298
- DOI: 10.1128/JVI.72.11.8813-8819.1998
Vaccinia virus protein synthesis has a low requirement for the intact translation initiation factor eIF4F, the cap-binding complex, within infected cells
Abstract
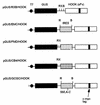
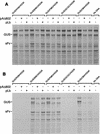
The role of the cap-binding complex, eIF4F, in the translation of vaccinia virus mRNAs has been analyzed within infected cells. Plasmid DNAs, which express dicistronic mRNAs containing a picornavirus internal ribosome entry site, produced within vaccinia virus-infected cells both beta-glucuronidase and a cell surface-targeted single-chain antibody (sFv). Cells expressing sFv were selected from nonexpressing cells, enabling analysis of protein synthesis specifically within the transfected cells. Coexpression of poliovirus 2A or foot-and-mouth disease virus Lb proteases, which cleaved translation initiation factor eIF4G, greatly inhibited cap-dependent protein (beta-glucuronidase) synthesis. Under these conditions, internal ribosome entry site-directed expression of sFv continued and cell selection was maintained. Furthermore, vaccinia virus protein synthesis persisted in the selected cells containing cleaved eIF4G. Thus, late vaccinia virus protein synthesis has a low requirement for the intact cap-binding complex eIF4F. This may be attributed to the short unstructured 5' noncoding regions of the vaccinia virus mRNAs, possibly aided by the presence of poly(A) at both 5' and 3' termini.
Figures
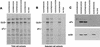


References
-
- Abrams C C, King A M Q, Belsham G J. Assembly of foot-and-mouth disease virus empty capsids synthesized by a vaccinia virus expression system. J Gen Virol. 1995;76:3089–3098. - PubMed
-
- Belsham G J, Brangwyn J K, Ryan M D, Abrams C C, King A M Q. Intracellular expression and processing of foot-and-mouth disease virus capsid precursors using vaccinia virus vectors: influence of the L protease. Virology. 1990;176:524–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

