African swine fever virus is enveloped by a two-membraned collapsed cisterna derived from the endoplasmic reticulum
- PMID: 9765444
- PMCID: PMC110316
- DOI: 10.1128/JVI.72.11.8988-9001.1998
African swine fever virus is enveloped by a two-membraned collapsed cisterna derived from the endoplasmic reticulum
Abstract
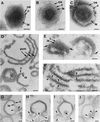
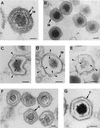
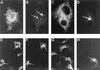
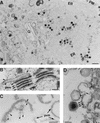
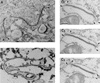
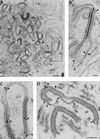
During the cytoplasmic maturation of African swine fever virus (ASFV) within the viral factories, the DNA-containing core becomes wrapped by two shells, an inner lipid envelope and an outer icosahedral capsid. We have previously shown that the inner envelope is derived from precursor membrane-like structures on which the capsid layer is progressively assembled. In the present work, we analyzed the origin of these viral membranes and the mechanism of envelopment of ASFV. Electron microscopy studies on permeabilized infected cells revealed the presence of two tightly apposed membranes within the precursor membranous structures as well as polyhedral assembling particles. Both membranes could be detached after digestion of intracellular virions with proteinase K. Importantly, membrane loop structures were observed at the ends of open intermediates, which suggests that the inner envelope is derived from a membrane cisterna. Ultraestructural and immunocytochemical analyses showed a close association and even direct continuities between the endoplasmic reticulum (ER) and assembling virus particles at the bordering areas of the viral factories. Such interactions become evident with an ASFV recombinant that inducibly expresses the major capsid protein p72. In the absence of the inducer, viral morphogenesis was arrested at a stage at which partially and fully collapsed ER cisternae enwrapped the core material. Together, these results indicate that ASFV, like the poxviruses, becomes engulfed by a two-membraned collapsed cisterna derived from the ER.
Figures



References
-
- Almeida J D, Waterson A P, Plowright W. The morphological characteristics of African swine fever virus and its resemblance to Tipula iridescent virus. Arch Gesamte Virusforsch. 1967;20:392–396. - PubMed
-
- Breese S S, Jr, DeBoer C J. Electron microscope observation of African swine fever virus in tissue culture cells. Virology. 1966;28:420–428. - PubMed
-
- Brookes S M, Dixon L K, Parkhouse R M E. Assembly of African swine fever virus: quantitative ultrastructural analysis in vitro and in vivo. Virology. 1996;224:84–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

