Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients
- PMID: 9765449
- PMCID: PMC110321
- DOI: 10.1128/JVI.72.11.9045-9053.1998
Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients
Abstract
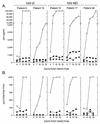
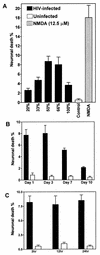
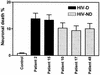
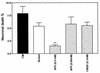
Human immunodeficiency virus type 1 (HIV-1) infection of the brain results in viral replication primarily in macrophages and microglia. Despite frequent detection of viral genome and proteins in the brains of AIDS patients with and without HIV dementia, only 20% of AIDS patients become demented. To investigate the role of viral envelope gene variation in the occurrence of dementia, we examined regions of variability in the viral envelope gene isolated from brains of AIDS patients. Brain-derived HIV-1 V1-V2 envelope sequences from seven demented and six nondemented AIDS patients displayed significant sequence differences between clinical groups, and by phylogenetic analysis, sequences from the demented group showed clustering. Infectious recombinant viruses containing brain-derived V3 sequences from both clinical groups were macrophagetropic, and viruses containing brain-derived V1, V2, and V3 sequences from both clinical groups spread efficiently in macrophages. In an indirect in vitro neurotoxicity assay using supernatant fluid from HIV-1-infected macrophages, recombinant viruses from demented patients induced greater neuronal death than viruses from nondemented patients. Thus, the HIV-1 envelope diversity observed in these patient groups appeared to influence the release of neurotoxic molecules from macrophages and might account in part for the variability in occurrence of dementia in AIDS patients.
Figures



References
-
- Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J C, Christov V I, Dawson T M, Dawson V L. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. - PubMed
-
- Barks J D E, Liu X-H, Sun R, Silverstein F S. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1996;76:397–409. - PubMed
-
- Bell J E, Busuttil A, Ironside J W, Rebus S, Donaldson Y K, Simmonds P, Peutherer J F. Human immunodeficiency virus and the brain: investigations of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis. 1993;168:818–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

