Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity
- PMID: 9765471
- PMCID: PMC110343
- DOI: 10.1128/JVI.72.11.9233-9246.1998
Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity
Abstract
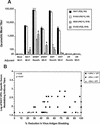
Virus-like particles (VLPs) are being evaluated as a candidate rotavirus vaccine. The immunogenicity and protective efficacy of different formulations of VLPs administered parenterally to rabbits were tested. Two doses of VLPs (2/6-, G3 2/6/7-, or P[2], G3 2/4/6/7-VLPs) or SA11 simian rotavirus in Freund's adjuvants, QS-21 (saponin adjuvant), or aluminum phosphate (AlP) were administered. Serological and mucosal immune responses were evaluated in all vaccinated and control rabbits before and after oral challenge with 10(3) 50% infective doses of live P[14], G3 ALA lapine rotavirus. All VLP- and SA11-vaccinated rabbits developed high levels of rotavirus-specific serum and intestinal immunoglobulin G (IgG) antibodies but not intestinal IgA antibodies. SA11 and 2/4/6/7-VLPs afforded similar but much higher mean levels of protection than 2/6/7- or 2/6-VLPs in QS-21. The presence of neutralizing antibodies to VP4 correlated (P < 0.001, r = 0.55; Pearson's correlation coefficient) with enhanced protection rates, suggesting that these antibodies are important for protection. Although the inclusion of VP4 resulted in higher mean protection levels, high levels of protection (87 to 100%) from infection were observed in individual rabbits immunized with 2/6/7- or 2/6-VLPs in Freund's adjuvants. Therefore, neither VP7 nor VP4 was absolutely required to achieve protection from infection in the rabbit model when Freund's adjuvant was used. Our results show that VLPs are immunogenic when administered parenterally to rabbits and that Freund's adjuvant is a better adjuvant than QS-21. The use of the rabbit model may help further our understanding of the critical rotavirus proteins needed to induce active protection. VLPs are a promising candidate for a parenterally administered subunit rotavirus vaccine.
Figures






References
-
- Andrew M E, Boyle D B, Coupar B E H, Reddy D, Bellamy A R, Both G W. Vaccinia-rotavirus VP7 recombinants protect mice against rotavirus-induced diarrhea. Vaccine. 1992;10:185–191. - PubMed
-
- Bertolotti-Ciarlet, A., S. E. Crawford, M. E. Conner, and M. K. Estes. Unpublished data.
-
- Burns J W, Chen D, Estes M K, Ramig R F. Biological and immunological characterization of a simian rotavirus SA11 variant with an altered genome segment 4. Virology. 1989;169:427–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

