The C-terminal half of the human immunodeficiency virus type 1 Gag precursor is sufficient for efficient particle assembly
- PMID: 9765481
- PMCID: PMC110353
- DOI: 10.1128/JVI.72.11.9313-9317.1998
The C-terminal half of the human immunodeficiency virus type 1 Gag precursor is sufficient for efficient particle assembly
Abstract
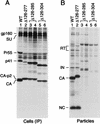
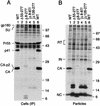
Human immunodeficiency virus type 1 particle assembly is directed by the Gag polyprotein Pr55(gag), the precursor for the matrix (MA), capsid (CA), and nucleocapsid proteins of the mature virion. We now show that CA sequences N terminal to the major homology region (MHR), which form a distinct domain, are dispensable for particle formation. However, slightly larger deletions which extend into the MHR severely impair particle production. Remarkably, a deletion which removed essentially all MA and CA sequences between the N-terminal myristyl anchor and the MHR reduced the yield of extracellular particles only moderately. Particle formation even exceeded wild-type levels when additional MA sequences, either from the N or the C terminus of the domain, were retained. We conclude that no distinct region between the myristyl anchor and the MHR is required for efficient particle assembly or release.
Figures


References
-
- Craven R C, Parent L J. Dynamic interactions of the Gag polyprotein. Curr Top Microbiol Immunol. 1996;214:65–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

