Legionella pneumophila catalase-peroxidases: cloning of the katB gene and studies of KatB function
- PMID: 9765568
- PMCID: PMC107585
- DOI: 10.1128/JB.180.20.5369-5374.1998
Legionella pneumophila catalase-peroxidases: cloning of the katB gene and studies of KatB function
Abstract
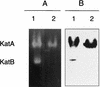
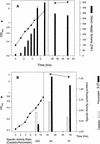
Legionella pneumophila, the causative organism of Legionnaires' pneumonia, is spread by aerosolization from man-made reservoirs, e.g. , water cooling towers and air conditioning ducts, whose nutrient-poor conditions are conducive to entrance into stationary phase. Exposure to starvation conditions is known to induce several virulence traits in L. pneumophila. Since catalase-peroxidases have been extremely useful markers of the stationary-phase response in many bacterial species and may be an avenue for identifying virulence genes in L. pneumophila, an investigation of these enzymes was initiated. L. pneumophila was shown to contain two bifunctional catalase-peroxidases and to lack monofunctional catalase and peroxidase. The gene encoding the KatB catalase-peroxidase was cloned and sequenced, and lacZ fusion and null mutant strains were constructed. Null mutants in katB are delayed in the infection and lysis of cultured macrophage-like cell lines. KatB is similar to the KatG catalase-peroxidase of Escherichia coli in its 20-fold induction during exponential growth and in playing a role in resistance to hydrogen peroxide. Analysis of the changes in katB expression and in the total catalase and peroxidase activity during growth indicates that the 8- to 10-fold induction of peroxidase activity that occurs in stationary phase is attributable to KatA, the second L. pneumophila catalase-peroxidase.
Figures



References
-
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;108:121–126. - PubMed
-
- Bandyopadhyay, P., and H. Steinman. 1998. Unpublished data.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

