Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product
- PMID: 9765580
- PMCID: PMC107597
- DOI: 10.1128/JB.180.20.5454-5457.1998
Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product
Abstract
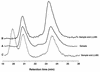
Recent studies of anaerobic toluene catabolism have demonstrated a novel reaction for anaerobic hydrocarbon activation: the addition of the methyl carbon of toluene to fumarate to form benzylsuccinate. In vitro studies of the anaerobic benzylsuccinate synthase reaction indicate that the H atom abstracted from the toluene methyl group during addition to fumarate is retained in the succinyl moiety of benzylsuccinate. Based on structural studies of benzylsuccinate formed during anaerobic, in vitro assays with denitrifying, toluene-mineralizing strain T, we now report the following characteristics of the benzylsuccinate synthase reaction: (i) it is highly stereospecific, resulting in >95% formation of the (+)-benzylsuccinic acid enantiomer [(R)-2-benzyl-3-carboxypropionic acid], and (ii) active benzylsuccinate synthase does not contain an abstracted methyl H atom from toluene at the beginning or at the end of a catalytic cycle.
Figures
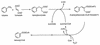
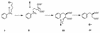
References
-
- Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

