From ab initio quantum mechanics to molecular neurobiology: a cation-pi binding site in the nicotinic receptor
- PMID: 9770444
- PMCID: PMC22789
- DOI: 10.1073/pnas.95.21.12088
From ab initio quantum mechanics to molecular neurobiology: a cation-pi binding site in the nicotinic receptor
Abstract
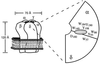
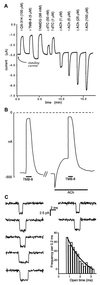
The nicotinic acetylcholine receptor is the prototype ligand-gated ion channel. A number of aromatic amino acids have been identified as contributing to the agonist binding site, suggesting that cation-pi interactions may be involved in binding the quaternary ammonium group of the agonist, acetylcholine. Here we show a compelling correlation between: (i) ab initio quantum mechanical predictions of cation-pi binding abilities and (ii) EC50 values for acetylcholine at the receptor for a series of tryptophan derivatives that were incorporated into the receptor by using the in vivo nonsense-suppression method for unnatural amino acid incorporation. Such a correlation is seen at one, and only one, of the aromatic residues-tryptophan-149 of the alpha subunit. This finding indicates that, on binding, the cationic, quaternary ammonium group of acetylcholine makes van der Waals contact with the indole side chain of alpha tryptophan-149, providing the most precise structural information to date on this receptor. Consistent with this model, a tethered quaternary ammonium group emanating from position alpha149 produces a constitutively active receptor.
Figures
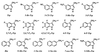

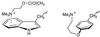
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

