Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene
- PMID: 9770464
- PMCID: PMC22809
- DOI: 10.1073/pnas.95.21.12202
Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene
Abstract
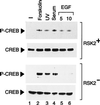
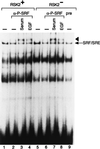
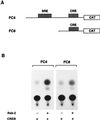
Activation by growth factors of the Ras-dependent signaling cascade results in the induction of p90 ribosomal S6 kinases (p90(rsk)). These are translocated into the nucleus upon phosphorylation by mitogen-activated protein kinases, with which p90(rsk) are physically associated in the cytoplasm. In humans there are three isoforms of the p90(rsk) family, Rsk-1, Rsk-2, and Rsk-3, which are products of distinct genes. Although these isoforms are structurally very similar, little is known about their functional specificity. Recently, mutations in the Rsk-2 gene have been associated with the Coffin-Lowry syndrome (CLS). We have studied a fibroblast cell line established from a CLS patient that bears a nonfunctional Rsk-2. Here we document that in CLS fibroblasts there is a drastic attenuation in the induced Ser-133 phosphorylation of transcription factor CREB (cAMP response element-binding protein) in response to epidermal growth factor stimulation. The effect is specific, since response to serum, cAMP, and UV light is unaltered. Furthermore, epidermal growth factor-induced expression of c-fos is severely impaired in CLS fibroblasts despite normal phosphorylation of serum response factor and Elk-1. Finally, coexpression of Rsk-2 in transfected cells results in the activation of the c-fos promoter via the cAMP-responsive element. Thus, we establish a link in the transduction of a specific growth factor signal to changes in gene expression via the phosphorylation of CREB by Rsk-2.
Figures



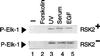
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

