Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction
- PMID: 9770465
- PMCID: PMC22810
- DOI: 10.1073/pnas.95.21.12208
Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction
Abstract
Nuclear receptors regulate metabolic pathways in response to changes in the environment by appropriate alterations in gene expression of key metabolic enzymes. Here, a computational search approach based on iteratively built hidden Markov models of nuclear receptors was used to identify a human nuclear receptor, termed hPAR, that is expressed in liver and intestines. hPAR was found to be efficiently activated by pregnanes and by clinically used drugs including rifampicin, an antibiotic known to selectively induce human but not murine CYP3A expression. The CYP3A drug-metabolizing enzymes are expressed in gut and liver in response to environmental chemicals and clinically used drugs. Interestingly, hPAR is not activated by pregnenolone 16alpha-carbonitrile, which is a potent inducer of murine CYP3A genes and an activator of the mouse receptor PXR.1. Furthermore, hPAR was found to bind to and trans-activate through a conserved regulatory sequence present in human but not murine CYP3A genes. These results provide evidence that hPAR and PXR.1 may represent orthologous genes from different species that have evolved to regulate overlapping target genes in response to pharmacologically distinct CYP3A activators, and have potential implications for the in vitro identification of drug interactions important to humans.
Figures

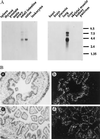
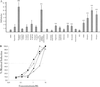

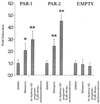
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

